Selective androgen receptor modulator
| Selective androgen receptor modulator | |
|---|---|
| Drug class | |
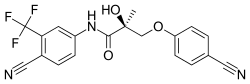 Enobosarm (ostarine), a nonsteroidal SARM which is or was under investigation for potential medical use. | |
| Class identifiers | |
| Synonyms | Partial androgens |
| Use | Hypogonadism; Osteopenia; Osteoporosis; Sarcopenia; Cachexia; |
| Biological target | Androgen receptor |
| Chemical class | Steroidal; Nonsteroidal |
| In Wikidata | |
Selective Androgen Receptor Modulators or SARMs are a novel class of androgen receptor ligands. In the late 1990s researchers were successful in finding the first nonsteroidal SARM, it was the discovery of an analog to bicalutamide; the search for these new drug candidates was driven by the undesirable side effects that come with androgenic-anabolic steroids.[1] They are intended to have the same kind of effects as androgenic drugs, such as anabolic-androgenic steroids, but be more selective in their action.[2] SARMs signify a new era of tissue-selective androgens with an unknown clinical application that could benefit the treatment of diseases. [3]
Comparison to testosterone
Currently used androgens for male hormone replacement therapy are typically injectable or skin delivery formulations of testosterone or testosterone esters. Injectable forms of testosterone esters (such as testosterone enanthate, propionate, or cypionate) produce undesirable fluctuations in testosterone blood levels, with overly high levels shortly after injection and overly low afterward. Skin patches do provide a better blood level profile of testosterone, but skin irritation and daily application still limit their usefulness.
SARMs provide the ability to design molecules that can be delivered orally, but that selectively target the androgen receptors in different tissues differently. The goal of research in this area is to allow a customized response: Tissues that are the target of the therapy will respond as they would to testosterone; other tissues where undesirable side-effects are produced will not.
None of the SARMs yet developed are truly selective for anabolic effects in muscle or bone tissues without producing any androgenic effects in tissues such as the prostate gland; however, several nonsteroidal androgens show a ratio of anabolic to androgenic effects of greater than 3:1 and up to as much as 90:1 (RAD-140), compared to testosterone, which has a ratio of 1:1.[4][5][6]
Research is continuing into more potent and selective SARMs, as well as optimizing characteristics such as oral bioavailability and increased half-life in vivo, and seeing as the first tissue-selective SARMs were only demonstrated in 2003, the compounds tested so far represent only the first generation of SARMs and future development may produce more selective agents compared to those available at present.[8][9][10]
Selectivity in men
For example, if the target is bone growth in elderly men with osteopenia or osteoporosis, but with no overt signs of hypogonadism, a SARM targeting bone and muscle tissue but with lesser activity on the prostate or testes would be more desirable.[11]
Selectivity in women
A SARM for women [12] would ideally stimulate bone retention, or libido and other function that androgens can influence, without negative side-effects such as development of male sex characteristics (virilization), increased LDL/HDL ratios, liver dysfunction, and so forth.[13]
Examples
Clinical testing

- Enobosarm (Ostarine, MK-2866, GTx-024, S-22) – One of the most popular SARMs, affects both muscle and bone, intended mainly for osteoporosis but also general treatment for andropause and reversing muscle sarcopenia in the elderly and for cachexia in cancer patients.[14]
- BMS-564,929 – Mainly affects muscle growth, intended as general treatment for symptoms of andropause
- LGD-4033 (Ligandrol) – pharmacological profile similar to that of enobosarm.
Pre-clinical
Abandoned drug candidates
- Acetothiolutamide – High-affinity AR full agonist in vitro, but very low activity in vivo due to poor pharmacokinetics[19]
- Andarine ("S-4")[20] – Partial agonist, intended mainly for treatment of benign prostatic hypertrophy
- LG-121071[21][22]
- TFM-4AS-1
- YK-11
Availability
In 2013, some supplement companies began selling various SARMs as supplements, in purported violation of both the Food and Drug Administration's Dietary Supplement Health and Education Act of 1994 (DSHEA) and the intellectual rights of the patent holders of the compounds.[23] In 2017 it was found that many of the supplements being sold claiming to be SARMs do not actually contain the chemical in question.[24] In reality, only 52% of the products contained any traces of SARMs at all.[25]
In October 2017, the Food and Drug Administration issued warning letters to three supplement companies notifying them that SARMS are classed as unapproved drugs and can cause potential adverse side effects associated including cardiovascular and liver damage.[26]
Use in sports
In 2015, quarterback of the Florida Gators, Will Grier, allegedly tested positive for Ligandrol, a claim that the University of Florida denies.[27]
In 2017, Joakim Noah was banned for twenty games by the NBA for testing positive for Ligandrol.[28]
Sean O'Malley, the American mixed martial artist who competes in the Bantamweight division of Ultimate Fighting Championship, was temporarily suspended by the Nevada State Athletic Commission in June 2019 after his sample tested positive for Enobosarm ahead of his fight against Marlon Vera at UFC 239 on July 6 in Las Vegas. [29]
In July 2019, tennis player Beatriz Haddad Maia from Brazil received a provisional suspension after she tested positive for selective androgen receptor modulators. [30]
Shayna Jack, the Australian swimmer, was forced to withdraw in July 2019 from the national squad before the world championships in Gwangju, South Korea after she tested positive for Ligandrol. [31]
The British relay team was stripped of their Tokyo Olympic silver medal after CJ Ujah, tested positive for two SARMs after the race. The banned substances discovered in his system were enobosarm (Ostarine) and S-23.
See also
References
- ↑ Thevis, M; Schanzer, W. (2010). Doping in Sports. Vol. 195. Springer. pp. 100–120. ISBN 978-3-540-79087-7.
- ↑ Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, Hwang DJ, Dalton JT, Miller DD (June 2009). "Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit". Journal of Medicinal Chemistry. 52 (12): 3597–617. doi:10.1021/jm900280m. PMID 19432422.
- ↑ Narayanan, Ramesh (April 2018). ""Development of Selective Androgen Receptor Modulators (SARMs)."". Molecular and Cellular Endocrinology (Molecular and Cellular Endocrinology, vol. 465 ed.). Elsevier BV. 465: 134–142. doi:10.1016/j.mce.2017.06.013. PMC 5896569. PMID 28624515. Retrieved 30 October 2020.
- ↑ Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT (March 2003). "Pharmacodynamics of selective androgen receptor modulators". The Journal of Pharmacology and Experimental Therapeutics. 304 (3): 1334–40. doi:10.1124/jpet.102.040840. PMC 2040238. PMID 12604714.
- ↑ Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T, Miyakawa M, Amano S, Sumita Y, Oguro N (November 2003). "Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis". Biological & Pharmaceutical Bulletin. 26 (11): 1563–9. doi:10.1248/bpb.26.1563. PMID 14600402.
- ↑ Ostrowski J, Kuhns JE, Lupisella JA, Manfredi MC, Beehler BC, Krystek SR, Bi Y, Sun C, Seethala R, Golla R, Sleph PG, Fura A, An Y, Kish KF, Sack JS, Mookhtiar KA, Grover GJ, Hamann LG (January 2007). "Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats". Endocrinology. 148 (1): 4–12. doi:10.1210/en.2006-0843. PMID 17008401.
- ↑ Aethyta (2015-10-19), English: Structure of RAD140., retrieved 2017-09-21
- ↑ Manfredi MC, Bi Y, Nirschl AA, Sutton JC, Seethala R, Golla R, Beehler BC, Sleph PG, Grover GJ, Ostrowski J, Hamann LG (August 2007). "Synthesis and SAR of tetrahydropyrrolo[1,2-b][1,2,5]thiadiazol-2(3H)-one 1,1-dioxide analogues as highly potent selective androgen receptor modulators". Bioorganic & Medicinal Chemistry Letters. 17 (16): 4487–90. doi:10.1016/j.bmcl.2007.06.007. PMID 17574413.
- ↑ Zhang X, Li X, Allan GF, Sbriscia T, Linton O, Lundeen SG, Sui Z (August 2007). "Design, synthesis, and in vivo SAR of a novel series of pyrazolines as potent selective androgen receptor modulators". Journal of Medicinal Chemistry. 50 (16): 3857–69. doi:10.1021/jm0613976. PMID 17636947.
- ↑ Long YO, Higuchi RI, Caferro TR, Lau TL, Wu M, Cummings ML, Martinborough EA, Marschke KB, Chang WY, López FJ, Karanewsky DS, Zhi L (May 2008). "Selective androgen receptor modulators based on a series of 7H-[1,4]oxazino[3,2-g]quinolin-7-ones with improved in vivo activity". Bioorganic & Medicinal Chemistry Letters. 18 (9): 2967–71. doi:10.1016/j.bmcl.2008.03.062. PMID 18400499.
- ↑ Ke HZ, Wang XN, O'Malley J, Lefker B, Thompson DD (2005). "Selective androgen receptor modulators--prospects for emerging therapy in osteoporosis?" (PDF). Journal of Musculoskeletal & Neuronal Interactions. 5 (4): 355. PMID 16340136.
- ↑ "SARM for women". 2020. Archived from the original on 2020-06-10.
- ↑ Negro-Vilar A (October 1999). "Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium". The Journal of Clinical Endocrinology and Metabolism. 84 (10): 3459–62. doi:10.1210/jc.84.10.3459. PMID 10522980.
- ↑ M.S. Steiner; et al. (June 2010). "Effect of GTx-024, a selective androgen receptor modulator (SARM), on stair climb and quality of life (QOL) in patients with cancer cachexia". J Clin Oncol. 28 (1534). doi:10.1200/jco.2010.28.15_suppl.9147.
- ↑ Piu F, Gardell LR, Son T, Schlienger N, Lund BW, Schiffer HH, Vanover KE, Davis RE, Olsson R, Bradley SR (March 2008). "Pharmacological characterization of AC-262536, a novel selective androgen receptor modulator". The Journal of Steroid Biochemistry and Molecular Biology. 109 (1–2): 129–37. doi:10.1016/j.jsbmb.2007.11.001. PMID 18164613. S2CID 25172405.
- ↑ Vajda EG, López FJ, Rix P, Hill R, Chen Y, Lee KJ, O'Brien Z, Chang WY, Meglasson MD, Lee YH (February 2009). "Pharmacokinetics and pharmacodynamics of LGD-3303 [9-chloro-2-ethyl-1-methyl-3-(2,2,2-trifluoroethyl)-3H-pyrrolo-[3,2-f]quinolin-7(6H)-one], an orally available nonsteroidal-selective androgen receptor modulator". The Journal of Pharmacology and Experimental Therapeutics. 328 (2): 663–70. doi:10.1124/jpet.108.146811. PMID 19017848. S2CID 22107491.
- ↑ Jones A, Chen J, Hwang DJ, Miller DD, Dalton JT (January 2009). "Preclinical characterization of a (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide: a selective androgen receptor modulator for hormonal male contraception". Endocrinology. 150 (1): 385–95. doi:10.1210/en.2008-0674. PMC 2630904. PMID 18772237.
- ↑ Miller CP, Shomali M, Lyttle CR, O'Dea LS, Herendeen H, Gallacher K, Paquin D, Compton DR, Sahoo B, Kerrigan SA, Burge MS, Nickels M, Green JL, Katzenellenbogen JA, Tchesnokov A, Hattersley G (February 2011). "Design, Synthesis, and Preclinical Characterization of the Selective Androgen Receptor Modulator (SARM) RAD140". ACS Medicinal Chemistry Letters. 2 (2): 124–9. doi:10.1021/ml1002508. PMC 4018048. PMID 24900290.
- ↑ Yin D, Xu H, He Y, Kirkovsky LI, Miller DD, Dalton JT (March 2003). "Pharmacology, pharmacokinetics, and metabolism of acetothiolutamide, a novel nonsteroidal agonist for the androgen receptor". The Journal of Pharmacology and Experimental Therapeutics. 304 (3): 1323–33. doi:10.1124/jpet.102.040832. PMID 12604713. S2CID 6816508.
- ↑ Kearbey JD, Gao W, Narayanan R, Fisher SJ, Wu D, Miller DD, Dalton JT (February 2007). "Selective Androgen Receptor Modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats". Pharmaceutical Research. 24 (2): 328–35. doi:10.1007/s11095-006-9152-9. PMC 2039878. PMID 17063395.
- ↑ Hamann LG, Mani NS, Davis RL, Wang XN, Marschke KB, Jones TK (January 1999). "Discovery of a potent, orally active, nonsteroidal androgen receptor agonist: 4-ethyl-1,2,3,4-tetrahydro-6- (trifluoromethyl)-8-pyridono[5,6-g]- quinoline (LG121071)". Journal of Medicinal Chemistry. 42 (2): 210–2. doi:10.1021/jm9806648. PMID 9925725.
- ↑ Gao W, Kim J, Dalton JT (August 2006). "Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands". Pharmaceutical Research. 23 (8): 1641–58. doi:10.1007/s11095-006-9024-3. PMC 2072875. PMID 16841196.
- ↑ "SARMs: The Controversial Muscle-Builders of 2015". The PricePlow Blog. Retrieved 20 October 2015.
- ↑ Van Wagoner, Ryan M.; Eichner, Amy; Bhasin, Shalender; Deuster, Patricia A.; Eichner, Daniel (28 November 2017). "Chemical Composition and Labeling of Substances Marketed as Selective Androgen Receptor Modulators and Sold via the Internet". JAMA. 318 (20): 2004–2010. doi:10.1001/jama.2017.17069. PMC 5820696. PMID 29183075.
- ↑ "Recreational Use of Selective Androgen Receptor Modulators".
- ↑ Commissioner, Office of the. "FDA In Brief - FDA In Brief: FDA warns against using SARMs in body-building products". www.fda.gov. Retrieved 2018-09-03.
- ↑ Trahan, Kevin (12 October 2015). "Florida starting QB Will Grier suspended for at least 2015 after taking banned substance". SBnation.com. Retrieved 20 October 2015.
- ↑ "Knicks' Joakim Noah Suspended for Failing a Doping Test". New York Times. March 25, 2017.
- ↑ "O'Malley gets ban for trace amounts of substance". June 22, 2019. Retrieved 19 March 2020.
- ↑ "Haddad Maia gets provisional ban after failing dope test". July 24, 2019. Retrieved 19 March 2020.
- ↑ "Shayna Jack reveals banned substance Ligandrol was behind her doping suspension from swimming". July 28, 2019. Retrieved 19 March 2020.
