Amfetaminil
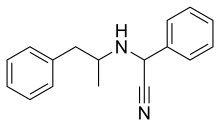
Amfetaminil (also known as amphetaminil, N-cyanobenzylamphetamine,[1] and AN-1; brand name Aponeuron) is a stimulant drug derived from amphetamine, which was developed in the 1970s and used for the treatment of obesity,[2] ADHD,[3][4] and narcolepsy.[5] It has largely been withdrawn from clinical use following problems with abuse.[6] The drug is a prodrug to amphetamine.[7][8]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.037.767 |
| Chemical and physical data | |
| Formula | C17H18N2 |
| Molar mass | 250.338 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Stereochemistry
Amfetaminil is a molecule with two stereogenic centers. Thus, four different stereoisomers exist:

- (R)-2-[(R)-1-Phenylpropan-2-ylamino]-2-phenylacetonitrile (CAS number 478392-08-4)
- (S)-2-[(S)-1-Phenylpropan-2-ylamino]-2-phenylacetonitrile (CAS number 478392-12-0)
- (R)-2-[(S)-1-Phenylpropan-2-ylamino]-2-phenylacetonitrile (CAS number 478392-10-8)
- (S)-2-[(R)-1-Phenylpropan-2-ylamino]-2-phenylacetonitrile (CAS number 478392-14-2)
Synthesis
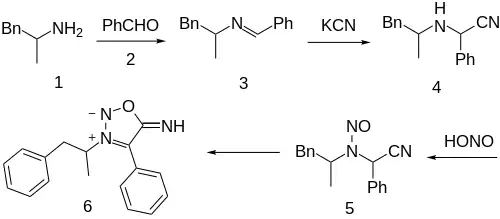
Schiff base formation between amphetamine (1) and benzaldehyde (2) gives benzalamphetamine [2980-02-1] (3). Nucleophilic attack of cyanide anion on the imine (c.f. Strecker reaction) gives amfetaminil (3). Finally, reaction with nitrous acid gives (5). The rearrangement to a Sydnone then occurs to give CID:88166659 (6). Feprosidnine is sans the phenyl group.
References
- Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 13–. ISBN 978-94-011-4439-1.
- Harris LS (June 1986). "The stimulants and hallucinogens under consideration: a brief overview of their chemistry and pharmacology". Drug and Alcohol Dependence. 17 (2–3): 107–18. doi:10.1016/0376-8716(86)90002-5. PMID 2874966.
- Meyer-Probst B, Vehreschild T (August 1976). "[Influencing the lack of concentration in hyperkinetic school children with Aponeuron]". Psychiatrie, Neurologie, und Medizinische Psychologie (in German). 28 (8): 491–9. PMID 1005547.
- Paclt I, Florian J, Brunclíková J, Růzicková I (May 1996). "[Effect of Aponeuron in the treatment of children with hyperkinetic syndrome]". Ceska a Slovenska Psychiatrie (in Czech). 92 Suppl 1: 41–57. PMID 8768943.
- Schlesser JL (1991). Drugs Available Abroad - A Guide to Therapeutic Drugs Approved Outside the US. Detroit: MEDEX Books.
- Winter E (September 1976). "[Drug abuse and dependence of the amphetamine type with special regard to Amphetaminil (Aponeuron(R))]". Psychiatrie, Neurologie, und Medizinische Psychologie (in German). 28 (9): 513–25. PMID 1005549.
- Dasgupta A (2 July 2012). Resolving Erroneous Reports in Toxicology and Therapeutic Drug Monitoring: A Comprehensive Guide. John Wiley & Sons. pp. 96–. ISBN 978-1-118-34785-0.
- AHC Media, LLC (17 March 2014). Pediatric Trauma Care II: A clinical reference for physicians and nurses caring for the acutely injured child. AHC Media, LLC. pp. 118–. ISBN 978-1-934863-59-6.
- Gorkin, V. Z.; Yashunskii, V. G.; Mashkovskii, M. D.; Al'tshuler, R. A.; Verevkina, I. V.; Kholodov, L. E. (1971). "Synthesis and pharmcological effects of some alkyl-, aryl, and aralkylsydnonimines". Journal of Medicinal Chemistry. 14 (10): 1013–1015. doi:10.1021/jm00292a042.
- Klosa, Josef (1963). "Synthese von Phenylisopropylaminoacetonitrilen". Journal für Praktische Chemie 20 (5-6): 275–278. doi:10.1002/prac.19630200509.
- Klosa, J., [The stability of amphetaminil. Syntheses with amphetaminil (author's transl)]. Arzneimittelforschung. 1975 Aug;25(8):1252-8. PMID: 1242355.
- Klosa J. [On the crystallisation of amphetaminil base into its hydrochloride salt (author's transl)]. Arzneimittelforschung. 1975;25(12):1863-4. PMID: 1243655.
- Beyer, K.H. et al, Deut. Apoth.-Ztg., 1971, 111, 677.
- Zhurnal Organicheskoi Khimii, , vol. 3, # 8 p. 1513 - 1518,1470 – 1474.
- Dipl-Chem Dr Josef Klosa, DE1112987 (1961); Chem. Abstr., 56: 3409d (1962).
- Klosa Josef Dr Dipl-Chem, CH417624 (1964).