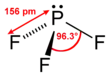Phosphorus trifluoride
Phosphorus trifluoride (formula PF3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls,[1] and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide.
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Phosphorus trifluoride Phosphorus(III) fluoride Trifluorophosphane Trifluoridophosphorus Perfluorophosphane | |||
| Other names
Trifluorophosphine Phosphorous fluoride TL-75 | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.098 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| PF3 | |||
| Molar mass | 87.968971 g/mol | ||
| Appearance | colorless gas | ||
| Density | 3.91 g/L, gas | ||
| Melting point | −151.5 °C (−240.7 °F; 121.6 K) | ||
| Boiling point | −101.8 °C (−151.2 °F; 171.3 K) | ||
| slow hydrolysis | |||
| Structure | |||
| Trigonal pyramidal | |||
| 1.03 D | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions |
Phosphorus trichloride Phosphorus tribromide Phosphorus triiodide Phosphane | ||
Other cations |
Nitrogen trifluoride Arsenic trifluoride Antimony trifluoride Bismuth trifluoride | ||
Related ligands |
Carbon monoxide | ||
Related compounds |
Phosphorus pentafluoride | ||
| Supplementary data page | |||
| Phosphorus trifluoride (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Physical properties
Phosphorus trifluoride has an F−P−F bond angle of approximately 96.3°. Gaseous PF3 has a standard enthalpy of formation of −945 kJ/mol (−226 kcal/mol). The phosphorus atom has a nuclear magnetic resonance chemical shift of 97 ppm (downfield of H3PO4).
Properties
Phosphorus trifluoride hydrolyzes especially at high pH, but it is less hydrolytically sensitive than phosphorus trichloride. It does not attack glass except at high temperatures, and anhydrous potassium hydroxide may be used to dry it with little loss. With hot metals, phosphides and fluorides are formed. With Lewis bases such as ammonia addition products (adducts) are formed, and PF3 is oxidized by oxidizing agents such as bromine or potassium permanganate.
As a ligand for transition metals, PF3 is a strong π-acceptor.[2] It forms a variety of metal complexes with metals in low oxidation states. PF3 forms several complexes for which the corresponding CO derivatives (see metal carbonyl) are unstable or nonexistent. Thus, Pd(PF3)4 is known, but Pd(CO)4 is not.[3][4][5] Such complexes are usually prepared directly from the related metal carbonyl compound, with loss of CO. However, nickel metal reacts directly with PF3 at 100 °C under 35 MPa pressure to form Ni(PF3)4, which is analogous to Ni(CO)4. Cr(PF3)6, the analogue of Cr(CO)6, may be prepared from dibenzenechromium:
platinum(0)-from-xtal-2008-3D-balls.png.webp) | platinum(0)-from-xtal-2008-3D-SF.png.webp) |
| Ball-and-stick model of [Pt(PF3)4] | Space-filling model of [Pt(PF3)4] |
Preparation
Phosphorus trifluoride is usually prepared from phosphorus trichloride via halogen exchange using various fluorides such as hydrogen fluoride, calcium fluoride, arsenic trifluoride, antimony trifluoride, or zinc fluoride:[6][7][8]
Biological activity
Phosphorus trifluoride is similar to carbon monoxide in that it is a gas which strongly binds to iron in hemoglobin, preventing the blood from absorbing oxygen.
References
- Chatt, J. (1950). "The Co-Ordinate Link in Chemistry". Nature. 165 (4199): 637–638. doi:10.1038/165637a0. PMID 15416738.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 494. ISBN 978-0-08-037941-8.
- Nicholls, D. (1973). Complexes and First-Row Transition Elements. London: Macmillan Press.
- Kruck, T. (1967). "Trifluorphosphin-Komplexe von Übergangsmetallen". Angewandte Chemie. 79 (1): 27–43. doi:10.1002/ange.19670790104.
- Clark, R. J.; Busch, M. A. (1973). "Stereochemical Studies of Metal Carbonyl-Phosphorus Trifluoride Complexes". Accounts of Chemical Research. 6 (7): 246–252. doi:10.1021/ar50067a005.
- Williams, A. A.; Parry, R. W.; Dess, H. (1957). "Phosphorus(III) Fluoride". Inorganic Syntheses. 5: 95–97. doi:10.1002/9780470132364.ch26.
- Dubrisay, R. (1956). Pascal, P. (ed.). Azote-Phosphore. Nouveau Traité de Chimie Minérale. Vol. 10. Paris, France: Masson. ISBN 978-2-225-57123-7.
- Clark, R. J.; Belefant, H.; Williamson, S. M. (1990). "Phosphorus Trifluoride". Inorganic Syntheses. 28: 310–315. doi:10.1002/9780470132593.ch77. ISBN 978-0-470-13259-3.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
Further reading
- Toy, A. D. F. (1973). The Chemistry of Phosphorus. Oxford, UK: Pergamon Press.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Lide, D. R., ed. (1990). Handbook of Chemistry and Physics (71st ed.). Ann Arbor, MI: CRC Press. ISBN 978-0-8493-0471-2.
- March, J. (1992). Advanced Organic Chemistry (4th ed.). New York: Wiley. p. 723. ISBN 978-0-471-60180-7.
- Stecher, P. G., ed. (1960). The Merck Index (7th ed.). Rahway, NJ, USA: Merck & Co.
- Holmes, R. R. (1960). "An Examination of the Basic Nature of the Trihalides of Phosphorus, Arsenic and Antimony". Journal of Inorganic and Nuclear Chemistry. 12 (3–4): 266–275. doi:10.1016/0022-1902(60)80372-7.