Durlobactam
 | |
| Clinical data | |
|---|---|
| Routes of administration | Intravenous |
| Drug class | Antibacterial, beta-lactamase inhibitor |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
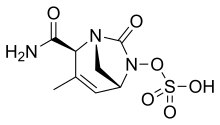
| Formula | C8H11N3O6S |
| Molar mass | 277.25 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Durlobactam is a beta-lactamase inhibitor used in combination with sulbactam to treat susceptible strains of bacteria in the genus Acinetobacter[1]
The combination therapy sulbactam/durlobactam was approved for medical use in the United States in May 2023.[1]
References
- 1 2 "FDA Approves New Treatment for Pneumonia Caused by Certain Difficult-to-Treat Bacteria". U.S. Food and Drug Administration (Press release). 24 May 2023. Retrieved 24 May 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Further reading
- Shapiro AB, Moussa SH, McLeod SM, Durand-Réville T, Miller AA (2021). "Durlobactam, a New Diazabicyclooctane β-Lactamase Inhibitor for the Treatment of Acinetobacter Infections in Combination With Sulbactam". Frontiers in Microbiology. 12: 709974. doi:10.3389/fmicb.2021.709974. PMC 8328114. PMID 34349751.
- Papp-Wallace KM, McLeod SM, Miller AA (May 2023). "Durlobactam, a Broad-Spectrum Serine β-lactamase Inhibitor, Restores Sulbactam Activity Against Acinetobacter Species". Clinical Infectious Diseases. 76 (Supplement_2): S194–S201. doi:10.1093/cid/ciad095. PMC 10150275. PMID 37125470.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.