Friedreich's ataxia
| Friedreich's ataxia | |
|---|---|
| Other names: Spinocerebellar ataxia, FRDA, FA | |
 | |
| Frataxin | |
| Specialty | Neurology |
| Symptoms | Difficulty walking, loss of sensation, impaired speech that worsens over time[1] |
| Complications | Cardiomyopathy, vision loss, hearing loss, scoliosis, diabetes[1] |
| Usual onset | 5–15 years[2] |
| Duration | Long-term[1] |
| Causes | Genetic (autosomal-recessive)[2] |
| Diagnostic method | Medical history, examination, genetic testing[2] |
| Differential diagnosis | Spinocerebellar ataxia, Charcot Marie Tooth disease, Ataxia telangiectasia[3] |
| Treatment | Supportive care[3] |
| Prognosis | Life expectancy ~ 37 years[4] |
| Frequency | 1 in 50,000 (United States)[2] |
Friedreich's ataxia (FRDA or FA) is a genetic disease that results in difficulty walking, loss of sensation, and impaired speech that worsens over time.[1] Mental functions are otherwise normal.[2] Symptoms generally start between 5 and 15 years of age.[2] As the disease progresses, many develop hypertrophic cardiomyopathy and lose their sight and hearing.[1] Other complications may include scoliosis and diabetes.[1]
The condition is inherited from a persons parents in an autosomal-recessive manner.[1] It occurs due to a mutations in the FXN gene on chromosome 9, which results in decreased production of a protein called frataxin.[1] This results in damage to highly active cells including neurons, heart cells, and pancreatic beta cells.[1] Diagnosis is often based on symptoms and confirmed by genetic testing.[2]
There is no specific treatment, though symptoms and complications may be managed.[2] This may include physiotherapy, mobility aids such as wheelchairs, and hearing aids.[1][2] FRDA shortens life expectancy to around 37 years,[4] though some live into their 60s or older.[2]
FRDA affects one in 50,000 people in the United States and is the most common inherited ataxia.[2] Rates are highest in Europe, the Middle East, South Asia, and North Africa.[3] The condition is named after German physician Nikolaus Friedreich, who first described it in 1863.[1] Research is ongoing with respect to a number of potential treatments.[4]
Signs and symptoms
Symptoms typically start between the ages of 5 and 15, but in late-onset FRDA, they may occur after age 25 years.[5] The symptoms are broad, but consistently involve gait and limb ataxia, dysarthria and loss of lower limb reflexes.[5] Non-neurological symptoms such as scoliosis, pes cavus, cardiomyopathy and diabetes are more frequent amongst the early-onset cases.[5]
Early
There is some variability in symptom frequency, onset and progression. 100% of individuals with FRDA develop neurological symptoms and >90% present with ataxia.[5] Cardiac issues are very common at diagnosis in individuals with typical onset FRDA and less common in late onset FRDA.[5] About 91% develop heart problems such as enlargement of the heart (up to dilated cardiomyopathy), symmetrical hypertrophy, heart murmurs, atrial fibrillation, fast heart rate, hypertrophic cardiomyopathy, and conduction defects. Scoliosis is present in about 60%. 7% of people with FRDA also have diabetes and having diabetes has an adverse impact on people with FA, especially those that show symptoms when young.[6][7]
Later
Dysarthria, spasticity, bladder and bowel symptoms develop later.[8] Vision loss is a later presentation and is progressive and can lead to functional blindness.[5] Advanced stages of disease are associated with supraventricular tachyarrhythmias, most commonly atrial fibrillation (AF).[5]
Other later stage symptoms can include, cerebellar effects such as nystagmus, fast saccadic eye movements, dysmetria and loss of coordination (truncal ataxia, and stomping gait).[5] The hearing can be impaired.[5] Symptoms can involve the dorsal column such as the loss of vibratory sensation and proprioceptive sensation.[5]
The progressive loss of coordination and muscle strength leads to the full-time use of a wheelchair. Most young people diagnosed with FRDA require mobility aids such as a cane, walker, or wheelchair by their childhood or early 20s.[9] The disease is progressive, with increasing staggering or stumbling gait and frequent falling. By the third decade, affected people lose the ability to stand or walk without assistance and require a wheelchair for mobility.[10]
Genetics
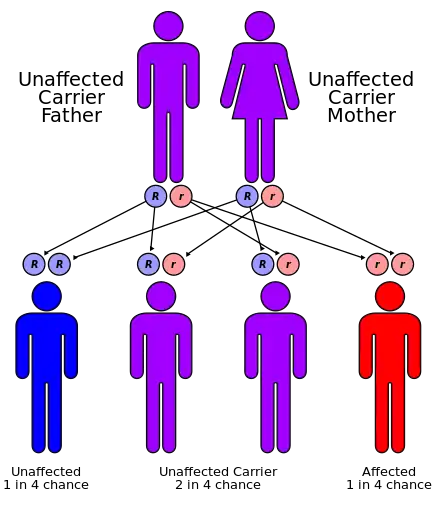
FRDA is an autosomal-recessive disorder that affects a gene (FXN) on chromosome 9, which produces an important protein called frataxin.[11]
In 96% of cases, the mutant FXN gene has 90–1,300 GAA trinucleotide repeat expansions in intron 1 of both alleles.[12] This expansion causes epigenetic changes and formation of heterochromatin near the repeat.[11] The length of the shorter GAA repeat is correlated with the age of onset and disease severity.[13] The formation of heterochromatin results in reduced transcription of the gene and low levels of frataxin.[14] People with FDRA might have 5-35% of the frataxin protein compared to healthy individuals. Heterozygous carriers of the mutant FXN gene have 50% lower frataxin levels, but this decrease is not enough to cause symptoms.[15]
In about 4% of cases, the disease is caused by a (missense, nonsense, or intronic) point mutation, with an expansion in one allele and a point mutation in the other.[16] A missense point mutation can have milder symptoms.[16] Depending on the point mutation, cells can produce no frataxin, nonfunctional frataxin, or frataxin that is not properly localized to the mitochondria.[17][18]
Pathophysiology
FRDA affects the nervous system, heart, pancreas, and other systems.[19][20]
Degeneration of nerve tissue in the spinal cord causes ataxia.[19] The sensory neurons essential for directing muscle movement of the arms and legs through connections with the cerebellum are particularly affected.[19] The disease primarily affects the spinal cord and peripheral nerves.
The spinal cord becomes thinner and nerve cells lose some myelin sheath.[19]The diameter of the spinal cord is smaller than that of unaffected individuals mainly due to smaller dorsal root ganglia.[20] The motor neurons of the spinal cord are affected to a lesser extent than sensory neurons.[19] In peripheral nerves, a loss of large myelinated sensory fibers occurs.[19]
Structures in the brain are also affected by FRDA, notably the dentate nucleus of the cerebellum.[20] The heart often develops some fibrosis, and over time, develops left-ventricle hypertrophy and dilatation of the left ventricle.[20]
Frataxin
The exact role of frataxin remains unclear.[21] Frataxin assists iron-sulfur protein synthesis in the electron transport chain to generate adenosine triphosphate, the energy molecule necessary to carry out metabolic functions in cells. It also regulates iron transfer in the mitochondria by providing a proper amount of reactive oxygen species (ROS) to maintain normal processes.[22] One result of frataxin deficiency is mitochondrial iron overload, which damages many proteins due to effects on cellular metabolism.[23]
Without frataxin, the energy in the mitochondria falls, and excess iron creates extra ROS, leading to further cell damage.[22] Low frataxin levels lead to insufficient biosynthesis of iron–sulfur clusters that are required for mitochondrial electron transport and assembly of functional aconitase and iron dysmetabolism of the entire cell.[23]
Diagnosis
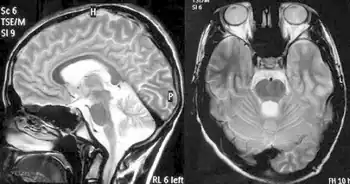
Balance difficulty, loss of proprioception, an absence of reflexes, and signs of other neurological problems are common signs from a physical examination.[10][25] Diagnostic tests are made to confirm a physical examination such as electromyogram, nerve conduction studies, electrocardiogram, echocardiogram, blood tests for elevated glucose levels and vitamin E levels, and scans such as X-ray radiograph for scoliosis.[26] MRI and CT scans of brain and spinal cord are done to rule out other neurological conditions.[27] Finally, a genetic test is conducted to confirm.[27]
Other diagnoses might include Charcot-Marie-Tooth types 1 and 2, ataxia with vitamin E deficiency, ataxia-oculomotor apraxia types 1 and 2, and other early-onset ataxias.[28]
Management
Physical therapists play a critical role in educating on correct posture, muscle use, and the identification and avoidance of features that aggravate spasticities such as tight clothing, poorly adjusted wheelchairs, pain, and infection.[29]
Rehabilitation
Physical therapy typically includes intensive motor coordination, balance, and stabilization training to preserve gains.[30] Low intensity strengthening exercises are incorporated to maintain functional use of the upper and lower extremities.[30] Stretching and muscle relaxation exercises can be prescribed to help manage spasticity and prevent deformities.[30] Other physical therapy goals include increased transfer and locomotion independence, muscle strengthening, increased physical resilience, "safe fall" strategy, learning to use mobility aids, learning how to reduce the body's energy expenditure, and developing specific breathing patterns.[30] Speech therapy can improve voice quality.[31]
Devices
Well-fitted orthoses can promote correct posture, support normal joint alignment, stabilize joints during walking, improve range of motion and gait, reduce spasticity, and prevent foot deformities and scoliosis.[9]
Functional electrical stimulation or transcutaneous nerve stimulation devices may alleviate symptoms.[9]
As progression of ataxia continues, assistive devices such as a cane, walker, or wheelchair may be required for mobility and independence. A standing frame can help reduce the secondary complications of prolonged use of a wheelchair.[32][33]
Medication and surgery
Cardiac abnormalities can be controlled with ACE inhibitors such as enalapril, ramipril, lisinopril, or trandolapril, sometimes used in conjunction with beta blockers. Affected people who also have symptomatic congestive heart failure may be prescribed eplerenone or digoxin to keep cardiac abnormalities under control.[9]
Surgery may correct deformities caused by abnormal muscle tone. Titanium screws and rods inserted in the spine help prevent or slow the progression of scoliosis. Surgery to lengthen the Achilles tendon can improve independence and mobility to alleviate equinus deformity.[9] An automated implantable cardioverter-defibrillator can be implanted after a severe heart failure.[9]
Prognosis
The disease evolves differently in different people.[32] In general, those diagnosed at a younger age or with longer GAA triplet expansions tend to have more severe symptoms.[9]
Congestive heart failure and abnormal heart rhythms are the leading causes of death,[34] but people with fewer symptoms can live into their 60s or older.[27]
Epidemiology
FRDA affects Indo-European populations. It is rare in East Asians, sub-Saharan Africans, and Native Americans. FRDA is the most prevalent inherited ataxia, affecting approximately 1 in 40,000 with European descent.[19] Males and females are affected equally. The estimated carrier prevalence is 1:100.[9] A 1990–1996 study of Europeans calculated the incidence rate was 2.8:100,000.[35] The prevalence rate of FRDA in Japan is 1:1,000,000.[36]
FRDA follows the same pattern as haplogroup R1b. Haplogroup R1b is the most frequently occurring paternal lineage in Western Europe. FRDA and Haplogroup R1b are more common in northern Spain, Ireland, and France, rare in Russia and Scandinavia, and follow a gradient through central and eastern Europe. A population carrying the disease went through a population bottleneck in the Franco-Cantabrian region during the last ice age.[37]
History

The condition is named after the 1860s German pathologist and neurologist, Nikolaus Friedreich.[38] Friedreich reported the disease in 1863 at the University of Heidelberg.[39][40][41] Further observations appeared in a paper in 1876.[42]
Frantz Fanon wrote his medical thesis on FRDA, in 1951.[43]
A 1984 Canadian study traced 40 cases to one common ancestral couple arriving in New France in 1634.[44]
FRDA was first linked to a GAA repeat expansion on chromosome 9 in 1996.[45]
Society and culture

The Cake Eaters is a 2007 independent drama film that stars Kristen Stewart as a young woman with FRDA.[46]
The Ataxian is a documentary that tells the story of Kyle Bryant, an athlete with FRDA who completes a long-distance bike race in an adaptive "trike" to raise money for research.[47]
Dynah Haubert spoke at the 2016 Democratic National Convention about supporting Americans with disabilities.[48]
Geraint Williams in an athlete affected by FRDA who is known for scaling Mount Kilimanjaro in an adaptive wheelchair.[49]
Shobhika Kalra is an activist with FRDA who helped build over 1000 wheelchair ramps across the United Arab Emirates in2018 to try to make Dubai fully wheelchair-friendly by 2020.[50]
Research
Active research is ongoing to find a treatment. Patients can enroll in a registry to make clinical trial recruiting easier. The Friedreich's Ataxia Global Patient Registry is the only worldwide registry of Friedreich's ataxia patients to characterize the symptoms and establish the rate of disease progression.[51]
As of May 2021, research continues along the following paths.
Mitochondrial function and oxidative stress
- Reata Pharmaceuticals developed RTA 408 (Omaveloxolone or Omav) to target activation of a transcriptional factor, Nrf2.[52] Nrf2 is decreased in FRDA cells.[53][54][55][56]
- PTC-743 (formerly EPI-743) is being developed by PTC Therapeutics. PTC-743 is a para-benzoquinone and targets the NAD(P)H dehydrogenase (quinone 1) (NQO1) enzyme to increase the biosynthesis of glutathione.[57]
- Retrotope is advancing RT001. RT001 is a deuterated synthetic homologue of ethyl linoleate, an essential omega-6 polyunsaturated fatty acid which is one of the major components of lipid membranes, particularly in mitochondria. Oxidation damage might be reduced if the polyunsaturated fatty acids in the lipids were made more rigid and less susceptible to oxidation by the replacement of hydrogen atoms with the heavy hydrogen isotope deuterium.[58]
Frataxin controlled metabolic pathways
- Dimethyl fumarate has been shown to increase frataxin levels in FRDA cells, mouse models, and humans. DMF showed an 85% increase in frataxin expression over 3 months in multiple sclerosis .[59]
Frataxin replacements or stabilizers
- EPO mimetics are orally available peptide imitations of erythropoietin. They are small molecules erythropoietin receptor agonists designed to activate the tissue-protective erythropoietin receptor.[60][61]
- Etravirine, an antiviral drug used to treat HIV, was found in a drug repositioning screening to increase frataxin levels in peripheral cells.[62] Fratagene Therapeutics is developing a small molecule called RNF126 to inhibit an enzyme which degrades frataxin.[63]
Frataxin gene expression
- Resveratrol might improve mitochondrial function.[64]
- Nicotinamide (vitamin B3) was found effective in preclinical FRDA models and well tolerated.[15]
- An RNA-based approach might unsilence the FXN gene and increase the expression of frataxin. Non-coding RNA (ncRNA) could be responsible for directing the localized epigenetic silencing of the FXN gene.
- Lentivirus-mediated delivery of the FXN gene has been shown to increase frataxin expression and prevent DNA damage in human and mouse fibroblasts.[65]
- CRISPR Therapeutics received a grant from the Friedreich's Ataxia Research Alliance to investigate gene editing as a potential treatment for the disease in 2017.[66]
References
- 1 2 3 4 5 6 7 8 9 10 11 Williams, CT; De Jesus, O (January 2021). "Friedreich Ataxia". PMID 33085346.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 6 7 8 9 10 11 "Friedreich Ataxia Fact Sheet". www.ninds.nih.gov. National Institute of Neurological Disorders and Stroke. Archived from the original on 23 January 2019. Retrieved 11 May 2021.
- 1 2 3 "Friedreich's Ataxia". NORD (National Organization for Rare Disorders). Archived from the original on 28 August 2021. Retrieved 11 May 2021.
- 1 2 3 Cook, A; Giunti, P (1 December 2017). "Friedreich's ataxia: clinical features, pathogenesis and management". British medical bulletin. 124 (1): 19–30. doi:10.1093/bmb/ldx034. PMID 29053830.
- 1 2 3 4 5 6 7 8 9 10 Cook, A.; Giunti, P. (2017). "Friedreich's ataxia: Clinical features, pathogenesis and management". British Medical Bulletin. 124 (1): 19–30. doi:10.1093/bmb/ldx034. PMC 5862303. PMID 29053830.
- ↑ McCormick, Ashley; Farmer, Jennifer; Perlman, Susan; Delatycki, Martin; Wilmot, George; Matthews, Katherine; Yoon, Grace; Hoyle, Chad; Subramony, Sub H.; Zesiewicz, Theresa; Lynch, David R.; McCormack, Shana E. (2017). "Impact of diabetes in the Friedreich ataxia clinical outcome measures study". Annals of Clinical and Translational Neurology. 4 (9): 622–631. doi:10.1002/acn3.439. PMC 5590524. PMID 28904984.
- ↑ Reetz, Kathrin; Dogan, Imis; Hohenfeld, Christian; Didszun, Claire; Giunti, Paola; Mariotti, Caterina; Durr, Alexandra; Boesch, Sylvia; Klopstock, Thomas; Rodríguez De Rivera Garrido, Francisco Javier; Schöls, Ludger; Giordano, Ilaria; Bürk, Katrin; Pandolfo, Massimo; Schulz, Jörg B.; EFACTS Study Group (2018). "Nonataxia symptoms in Friedreich Ataxia" (PDF). Neurology. 91 (10): e917–e930. doi:10.1212/WNL.0000000000006121. PMID 30097477. S2CID 51956258. Archived (PDF) from the original on 2020-05-05. Retrieved 2021-03-23.
- ↑ "Friedreich Ataxia Fact Sheet". Archived from the original on 23 January 2019. Retrieved 10 February 2019.
- 1 2 3 4 5 6 7 8 "Friedreich ataxia clinical management guidelines". Friedreich Ataxia Research Alliance (USA). 2014. Archived from the original on 20 October 2018. Retrieved 23 October 2018.
- 1 2 Parksinson, MH; Boesch, S; Nachbauer, W; Mariotti, C; Giunti, P (August 2013). "Clinical features of Friedreich's ataxia: classical and atypical phenotypes". Journal of Neurochemistry. 126 (Supplement 1): 103–17. doi:10.1111/jnc.12317. PMID 23859346. Archived from the original on 13 February 2021. Retrieved 5 February 2021.
- 1 2 Klockgether T (August 2011). "Update on degenerative ataxias". Current Opinion in Neurology. 24 (4): 339–45. doi:10.1097/WCO.0b013e32834875ba. PMID 21734495.
- ↑ Clark E, Johnson J, Dong YN, Mercado-Ayon, Warren N, Zhai M, McMillan E, Salovin A, Lin H, Lynch DR (Nov 2018). "Role of frataxin protein deficiency and metabolic dysfunction in Friedreich ataxia, an autosomal recessive mitochondrial disease". Neuronal Signaling. 2 (4): NS20180060. doi:10.1042/NS20180060. PMC 7373238. PMID 32714592.
- ↑ Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, et al. (October 1996). "Clinical and genetic abnormalities in patients with Friedreich's ataxia". The New England Journal of Medicine. 335 (16): 1169–75. doi:10.1056/nejm199610173351601. PMID 8815938.
- ↑ Montermini L, Andermann E, Labuda M, Richter A, Pandolfo M, Cavalcanti F, et al. (August 1997). "The Friedreich ataxia GAA triplet repeat: premutation and normal alleles". Human Molecular Genetics. 6 (8): 1261–6. doi:10.1093/hmg/6.8.1261. PMID 9259271.
- 1 2 Bürk K (2017). "Friedreich Ataxia: current status and future prospects". Cerebellum & Ataxias. 4: 4. doi:10.1186/s40673-017-0062-x. PMC 5383992. PMID 28405347.
- 1 2 Cossée M, Dürr A, Schmitt M, Dahl N, Trouillas P, Allinson P, et al. (February 1999). "Friedreich's ataxia: point mutations and clinical presentation of compound heterozygotes". Annals of Neurology. 45 (2): 200–6. doi:10.1002/1531-8249(199902)45:2<200::AID-ANA10>3.0.CO;2-U. PMID 9989622.
- ↑ Lazaropoulos M, Dong Y, Clark E, Greeley NR, Seyer LA, Brigatti KW, et al. (August 2015). "Frataxin levels in peripheral tissue in Friedreich ataxia". Annals of Clinical and Translational Neurology. 2 (8): 831–42. doi:10.1002/acn3.225. PMC 4554444. PMID 26339677.
- ↑ Galea CA, Huq A, Lockhart PJ, Tai G, Corben LA, Yiu EM, et al. (March 2016). "Compound heterozygous FXN mutations and clinical outcome in friedreich ataxia". Annals of Neurology. 79 (3): 485–95. doi:10.1002/ana.24595. PMID 26704351. S2CID 26709558.
- 1 2 3 4 5 6 7 Delatycki, Martin B.; Bidichandani, Sanjay I. (2019). "Friedreich ataxia- pathogenesis and implications for therapies". Neurobiology of Disease. 132: 104606. doi:10.1016/j.nbd.2019.104606. PMID 31494282. S2CID 201839487.
- 1 2 3 4 Hanson, Emily; Sheldon, Mark; Pacheco, Brenda; Alkubeysi, Mohammed; Raizada, Veena (2019). "Heart disease in Friedreich's ataxia". World Journal of Cardiology. 11 (1): 1–12. doi:10.4330/wjc.v11.i1.1. PMC 6354072. PMID 30705738.
- ↑ Maio, Nunziata; Jain, Anshika; Rouault, Tracey A. (2020). "Mammalian iron–sulfur cluster biogenesis: Recent insights into the roles of frataxin, acyl carrier protein and ATPase-mediated transfer to recipient proteins". Current Opinion in Chemical Biology. 55: 34–44. doi:10.1016/j.cbpa.2019.11.014. PMC 7237328. PMID 31918395.
- 1 2 Sahdeo S, Scott BD, McMackin MZ, Jasoliya M, Brown B, Wulff H, et al. (December 2014). "Dyclonine rescues frataxin deficiency in animal models and buccal cells of patients with Friedreich's ataxia". Human Molecular Genetics. 23 (25): 6848–62. doi:10.1093/hmg/ddu408. PMC 4245046. PMID 25113747.
- 1 2 "Role of Frataxin, a Protein Implicated in Friedreich Ataxia, in Making Iron-Sulfur Clusters♦". Journal of Biological Chemistry. 288 (52): 36787. 2013. doi:10.1074/jbc.P113.525857. S2CID 220291178.
- ↑ Pandolfo, Massimo (October 2008). "Friedreich ataxia". Archives of Neurology. 65 (10): 1296–1303. doi:10.1001/archneur.65.10.1296. ISSN 1538-3687. Archived from the original on 19 January 2022. Retrieved 23 May 2022.
- ↑ Corben, LA; Lynch, D; Pandolfo, M; Schulz, JB; Delatycki, MB; Clinical Management Guidelines Writing Group (November 2014). "Consensus clinical management guidelines for Friedreich ataxia". Orphanet Journal of Rare Diseases. 9: 184. doi:10.1186/s13023-014-0184-7. PMC 4280001. PMID 25928624.
- ↑ Brigatti KW, Deutsch EC, Lynch DR, Farmer JM (September 2012). "Novel diagnostic paradigms for Friedreich ataxia". Journal of Child Neurology. 27 (9): 1146–51. doi:10.1177/0883073812448440. PMC 3674546. PMID 22752491.
- 1 2 3 "Friedreich's Ataxia Fact Sheet". National Institute of Neurological Disorders and Stroke. Archived from the original on 26 August 2017. Retrieved 26 August 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Friedreich ataxia NIH page". NIH Rare diseases. Archived from the original on 31 March 2019. Retrieved 17 March 2019.
- ↑ Aranca TV, Jones TM, Shaw JD, Staffetti JS, Ashizawa T, Kuo SH, et al. (Feb 2016). "Emerging therapies in Friedreich's ataxia". Neurodegenerative Disease Management. 6 (1): 49–65. doi:10.2217/nmt.15.73. PMC 4768799. PMID 26782317.
- 1 2 3 4 Chien H, Barsottini O (10 December 2016). Chien HF, Barsottini OG (eds.). Movement Disorders Rehabilitation. Springer, Cham. pp. 83–95. doi:10.1007/978-3-319-46062-8. ISBN 978-3-319-46062-8.
- ↑ Lowit, Anja; Egan, Aisling; Hadjivassiliou, Marios (2020). "Feasibility and Acceptability of Lee Silverman Voice Treatment in Progressive Ataxias". The Cerebellum. 19 (5): 701–714. doi:10.1007/s12311-020-01153-3. PMC 7471180. PMID 32588316.
- 1 2 Ojoga F, Marinescu S (2013). "Physical Therapy and Rehabilitation for Ataxic Patients". Balneo Research Journal. 4 (2): 81–84. doi:10.12680/balneo.2013.1044.
- ↑ Leonardi L, Aceto MG, Marcotulli C, Arcuria G, Serrao M, Pierelli F, et al. (March 2017). "A wearable proprioceptive stabilizer for rehabilitation of limb and gait ataxia in hereditary cerebellar ataxias: a pilot open-labeled study". Neurological Sciences. 38 (3): 459–463. doi:10.1007/s10072-016-2800-x. PMID 28039539. S2CID 27569800.
- ↑ Doğan-Aslan M, Büyükvural-Şen S, Nakipoğlu-Yüzer GF, Özgirgin N (September 2018). "Demographic and clinical features and rehabilitation outcomes of patients with Friedreich ataxia: A retrospective study" (PDF). Turkish Journal of Physical Medicine and Rehabilitation. 64 (3): 230–238. doi:10.5606/tftrd.2018.2213. PMC 6657791. PMID 31453516. Archived (PDF) from the original on 30 October 2018. Retrieved 29 October 2018.
- ↑ Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, et al. (October 1996). "Clinical and genetic abnormalities in patients with Friedreich's ataxia". The New England Journal of Medicine. 335 (16): 1169–75. doi:10.1056/NEJM199610173351601. PMID 8815938.
- ↑ Kita K (December 1993). "[Spinocerebellar degeneration in Japan--the feature from an epidemiological study]". Rinsho Shinkeigaku = Clinical Neurology. 33 (12): 1279–84. PMID 8174325.
- ↑ Vankan P (August 2013). "Prevalence gradients of Friedreich's ataxia and R1b haplotype in Europe co-localize, suggesting a common Palaeolithic origin in the Franco-Cantabrian ice age refuge". Journal of Neurochemistry. 126 Suppl 1: 11–20. doi:10.1111/jnc.12215. PMID 23859338. S2CID 39343424.
- ↑ Nicolaus Friedreich at Who Named It?
- ↑ Friedreich N (1863). "Ueber degenerative Atrophie der spinalen Hinterstränge" [About degenerative atrophy of the spinal posterior column]. Arch Pathol Anat Phys Klin Med (in Deutsch). 26 (3–4): 391–419. doi:10.1007/BF01930976. S2CID 42991858.
- ↑ Friedreich N (1863). "Ueber degenerative Atrophie der spinalen Hinterstränge" [About degenerative atrophy of the spinal posterior column]. Arch Pathol Anat Phys Klin Med (in Deutsch). 26 (5–6): 433–459. doi:10.1007/BF01878006. S2CID 34515886.
- ↑ Friedreich N (1863). "Ueber degenerative Atrophie der spinalen Hinterstränge" [About degenerative atrophy of the spinal posterior column]. Arch Pathol Anat Phys Klin Med (in Deutsch). 27 (1–2): 1–26. doi:10.1007/BF01938516. S2CID 46459932.
- ↑ Friedreich N (1876). "Ueber Ataxie mit besonderer Berücksichtigung der hereditären Formen" [About ataxia with special reference to hereditary forms]. Arch Pathol Anat Phys Klin Med (in Deutsch). 68 (2): 145–245. doi:10.1007/BF01879049. S2CID 42155823. Archived from the original on 2019-08-27. Retrieved 2019-08-27.
- ↑ Adam Shatz, "Where Life Is Seized" Archived 12 January 2017 at the Wayback Machine, London Review of Books, 19 January 2017
- ↑ Barbeau A, Sadibelouiz M, Roy M, Lemieux B, Bouchard JP, Geoffroy G (November 1984). "Origin of Friedreich's disease in Quebec". The Canadian Journal of Neurological Sciences. 11 (4 Suppl): 506–9. doi:10.1017/S0317167100034971. PMID 6391645.
- ↑ Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, et al. (March 1996). "Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion". Science. 271 (5254): 1423–7. Bibcode:1996Sci...271.1423C. doi:10.1126/science.271.5254.1423. PMID 8596916. S2CID 20303793.
- ↑ Holden S (13 March 2009). "The Cake Eaters". The New York Times. Archived from the original on 28 August 2021. Retrieved 8 July 2009.
- ↑ "Devastating Diagnosis Pushes Local Man To Live Bigger". CBS Sacramento. 30 May 2015. Archived from the original on 16 June 2015. Retrieved 12 June 2015.
- ↑ "How the DNC Is Subtly Rebuking Donald Trump's Mockery of a Disabled Reporter". Slate. 27 July 2016. Archived from the original on 15 December 2018. Retrieved 14 December 2018.
- ↑ "Man with rare nerve condition climbs Mount Kilimanjaro to raise money for charity". ITV. Archived from the original on 15 December 2018. Retrieved 14 December 2018.
- ↑ "Shobhika Kalra: Meet the Dubai woman in wheelchair who helped build 1,000 ramps across UAE". GULF NEWS. Archived from the original on 15 December 2018. Retrieved 14 December 2018.
- ↑ "FA Global Patient Registry (FAGPR)". FA Global Patient Registry (FAGPR). Archived from the original on 27 April 2021. Retrieved 27 April 2021.
- ↑ Reisman SA, Lee CY, Meyer CJ, Proksch JW, Sonis ST, Ward KW (May 2014). "Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis". Radiation Research. 181 (5): 512–20. Bibcode:2014RadR..181..512R. doi:10.1667/RR13578.1. PMID 24720753. S2CID 23906747.
- ↑ Shan Y, Schoenfeld RA, Hayashi G, Napoli E, Akiyama T, Iodi Carstens M, et al. (November 2013). "Frataxin deficiency leads to defects in expression of antioxidants and Nrf2 expression in dorsal root ganglia of the Friedreich's ataxia YG8R mouse model". Antioxidants & Redox Signaling. 19 (13): 1481–93. doi:10.1089/ars.2012.4537. PMC 3797453. PMID 23350650..
- ↑ "A Phase 2 Study of the Safety, Efficacy, and Pharmacodynamics of RTA 408 in the Treatment of Friedreich's Ataxia (MOXIe)". 1 October 2020. Archived from the original on 3 April 2021. Retrieved 22 March 2021 – via clinicaltrials.gov.
- ↑ "FARA – Part 2 of the Phase II MOXIe study (RTA 408 or omaveloxolone)". www.curefa.org. Archived from the original on 2020-03-07. Retrieved 2021-03-22.
- ↑ Lynch DR, Farmer J, Hauser L, Blair IA, Wang QQ, Mesaros C, et al. (January 2019). "Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia". Annals of Clinical and Translational Neurology. 6 (1): 15–26. doi:10.1002/acn3.660. PMC 6331199. PMID 30656180.
- ↑ Enns GM, Kinsman SL, Perlman SL, Spicer KM, Abdenur JE, Cohen BH, et al. (January 2012). "Initial experience in the treatment of inherited mitochondrial disease with EPI-743". Molecular Genetics and Metabolism. 105 (1): 91–102. doi:10.1016/j.ymgme.2011.10.009. PMID 22115768.
- ↑ Indelicato E, Bosch S (2018). "Emerging therapeutics for the treatment of Friedreich's ataxia". Expert Opinion on Orphan Drugs. 6: 57–67. doi:10.1080/21678707.2018.1409109. S2CID 80157839.
- ↑ Jasoliya M, Sacca F, Sahdeo S, Chedin F, Pane C, Brescia Morra V, et al. (June 2019). "Dimethyl fumarate dosing in humans increases frataxin expression: A potential therapy for Friedreich's Ataxia". PLOS ONE. 14 (6): e0217776. Bibcode:2019PLoSO..1417776J. doi:10.1371/journal.pone.0217776. PMC 6546270. PMID 31158268.
- ↑ Miller JL, Rai M, Frigon NL, Pandolfo M, Punnonen J, Spencer JR (September 2017). "Erythropoietin and small molecule agonists of the tissue-protective erythropoietin receptor increase FXN expression in neuronal cells in vitro and in Fxn-deficient KIKO mice in vivo". Neuropharmacology. 123: 34–45. doi:10.1016/j.neuropharm.2017.05.011. PMID 28504123. S2CID 402724.
- ↑ "STATegics, Inc. Announces a New Grant from Friedreich's Ataxia Research Alliance" (PDF). Archived (PDF) from the original on 2019-04-09. Retrieved 2019-04-09.
- ↑ Alfedi G, Luffarelli R, Condò I, Pedini G, Mannucci L, Massaro DS, et al. (March 2019). "Drug repositioning screening identifies etravirine as a potential therapeutic for friedreich's ataxia". Movement Disorders. 34 (3): 323–334. doi:10.1002/mds.27604. PMID 30624801. S2CID 58567610.
- ↑ Benini M, Fortuni S, Condò I, Alfedi G, Malisan F, Toschi N, et al. (February 2017). "E3 Ligase RNF126 Directly Ubiquitinates Frataxin, Promoting Its Degradation: Identification of a Potential Therapeutic Target for Friedreich Ataxia". Cell Reports. 18 (8): 2007–2017. doi:10.1016/j.celrep.2017.01.079. PMC 5329121. PMID 28228265.
- ↑ "Jupiter Orphan Therapeutics, Inc. Enters into a Global Licensing Agreement with Murdoch Childrens Research Institute" (PDF). Archived (PDF) from the original on 2019-07-11. Retrieved 2019-04-08.
- ↑ Khonsari H, Schneider M, Al-Mahdawi S, Chianea YG, Themis M, Parris C, et al. (December 2016). "Lentivirus-meditated frataxin gene delivery reverses genome instability in Friedreich ataxia patient and mouse model fibroblasts". Gene Therapy. 23 (12): 846–856. doi:10.1038/gt.2016.61. PMC 5143368. PMID 27518705.
- ↑ Melão A (19 October 2017). "CRISPR Therapeutics Receives FARA Grant to Develop Gene Editing Therapies for Friedreich's Ataxia". Friedreich's Ataxia News. Archived from the original on 21 April 2019. Retrieved 21 April 2019.
External links
- Friedreich's Ataxia Global Patient Registry Archived 2021-04-10 at the Wayback Machine
- NIH's FRDA information page Archived 2021-11-23 at the Wayback Machine
| Classification | |
|---|---|
| External resources |
|