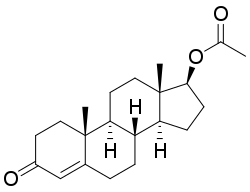
Testosterone acetate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Aceto-Sterandryl, Aceto-Testoviron, Amolisin, Androtest A, Deposteron, Farmatest, Perandrone A |
| Other names | Testosterone ethanoate; Testosterone 17β-acetate; Androst-4-en-17β-ol-3-one 17β-acetate |
| Routes of administration | Intramuscular injection |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.615 |
| Chemical and physical data | |
| Formula | C21H30O3 |
| Molar mass | 330.468 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Testosterone acetate (brand names Aceto-Sterandryl, Aceto-Testoviron, Amolisin, Androtest A, Deposteron, Farmatest, Perandrone A), or testosterone ethanoate, also known as androst-4-en-17β-ol-3-one 17β-acetate, is an androgen and anabolic steroid and a testosterone ester.[1][2][3] The drug was first described in 1936 and was one of the first androgen esters and esters of testosterone to be synthesized.[4][5]
See also
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 641–642. ISBN 978-1-4757-2085-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. ISBN 978-3-88763-075-1.
- ↑ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- ↑ Miescher K, Wettstein A, Tschopp E (1936). "The activation of the male sex hormones. II". Biochem. J. 30 (11): 1977–90. doi:10.1042/bj0301977. PMC 1263292. PMID 16746250.
- ↑ Parkes, A.S. (1936). "Increasing the Effectiveness of Testosterone". The Lancet. 228 (5899): 674–676. doi:10.1016/S0140-6736(00)80929-0. ISSN 0140-6736.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.