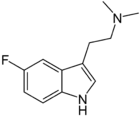
5-Fluoro-DMT
5-Fluoro-N,N-dimethyltryptamine (5-fluoro-DMT, 5F-DMT) is a tryptamine derivative related to compounds such as 5-bromo-DMT and 5-MeO-DMT.[1] Fluorination of psychedelic tryptamines either reduces or has little effect on 5-HT2A/C receptor affinity or intrinsic activity, although 6-fluoro-DET is inactive as a psychedelic despite acting as a 5-HT2A agonist (cf. lisuride), while 4-fluoro-5-methoxy-DMT is a much stronger agonist at 5-HT1A than 5-HT2A.[2][3]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H15FN2 |
| Molar mass | 206.264 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
See also
- 5-Fluoro-AMT
- 5-Fluoro-DET
- 5-Fluoro-MET
- 6-fluoro-AMT
- 6-Fluoro-DMT
- 4-fluoro-5-methoxy-DMT
- 6-Fluoro-DET(6-Fluoro-DET)
- O-4310
- It's worth noting that GR-159897 is based on the same structure.
References
- Chen CY, Senanayake CH, Bill TJ, Larsen RD, Verhoeven TR, Reider PJ (July 1994). "Improved Fischer indole reaction for the preparation of N, N-dimethyltryptamines: Synthesis of L-695,894, a potent 5-HT1D receptor agonist". The Journal of Organic Chemistry. 59 (13): 3738–3741. doi:10.1021/jo00092a046.
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE (November 2000). "Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines". Journal of Medicinal Chemistry. 43 (24): 4701–10. doi:10.1021/jm000339w. PMID 11101361.
- Rabin RA, Regina M, Doat M, Winter JC (May 2002). "5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens". Pharmacology, Biochemistry, and Behavior. 72 (1–2): 29–37. doi:10.1016/S0091-3057(01)00720-1. PMID 11900766. S2CID 6480715.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.