Inositol
Inositol, or more precisely myo-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues; it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and participates in osmoregulation.[2]
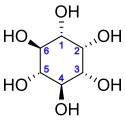 | |
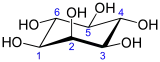 | |
 | |
| Names | |
|---|---|
| IUPAC name
myo-Inositol | |
| Systematic IUPAC name
(1R,2S,3r,4R,5S,6s)-Cyclohexane-1,2,3,4,5,6-hexol | |
| Other names
cis-1,2,3,5-trans-4,6-Cyclohexanehexol Cyclohexanehexol Mouse antialopecia factor Nucite Phaseomannite Phaseomannitol Rat antispectacled eye factor Scyllite (for the isomer scyllo-inositol) Vitamin B8 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.295 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g/mol |
| Density | 1.752 g/cm3 |
| Melting point | 225 to 227 °C (437 to 441 °F; 498 to 500 K) |
| Pharmacology | |
| A11HA07 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 143 °C (289 °F; 416 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is a sugar alcohol with half the sweetness of sucrose (table sugar). It is made naturally in the human body from glucose. A human kidney makes about two grams per day. Other tissues synthesize it too, and the highest concentration is in the brain, where it plays an important role in making other neurotransmitters and some steroid hormones bind to their receptors.[3]
Inositol is promoted as a dietary supplement in the management of polycystic ovary syndrome (PCOS). However, there is only evidence of very low quality for its efficacy in increasing fertility for IVF in women with PCOS.[4]
Overview
myo-Inositol plays an important role as the structural basis for a number of secondary messengers in eukaryotic cells, the various inositol phosphates. In addition, inositol serves as an important component of the structural lipids phosphatidylinositol (PI) and its various phosphates, the phosphatidylinositol phosphate (PIP) lipids.
Inositol or its phosphates and associated lipids are found in many foods, in particular fruit, especially cantaloupe and oranges.[5] In plants, the hexaphosphate of inositol, phytic acid or its salts, the phytates, serve as phosphate stores in seed, for example in nuts and beans.[6] Phytic acid also occurs in cereals with high bran content. Phytate is, however, not directly bioavailable to humans in the diet, since it is not digestible. Some food preparation techniques partly break down phytates to change this. However, inositol in the form of glycerophospholipids, as found in certain plant-derived substances such as lecithins, is well absorbed and relatively bioavailable.
myo-Inositol (free of phosphate) was once considered a member of the vitamin B complex, called Vitamin B8 in this context. However, because it is produced by the human body from glucose, it is not an essential nutrient.[7]
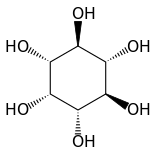
Isomers and structure
myo-Inositol is the biologically important form of cyclohexane-1,2,3,4,5,6-hexol. It is a meso compound, i.e. optically inactive because it has a plane of symmetry. It was formerly called meso-inositol, but because there are other meso isomers, myo-inositol is now the preferred name. Besides myo-inositol, the other naturally occurring stereoisomers are scyllo-, muco-, D-chiro-, L-chiro-, and neo-inositol, although they occur in minimal quantities in nature. The other possible isomers are allo-, epi-, and cis-inositol. As their names denote, L- and D-chiro inositol are the only pair of enantiomers (mirror-image forms). All the others are meso compounds.[8]
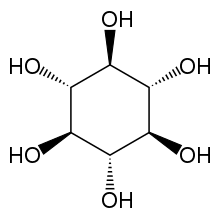
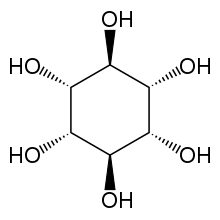
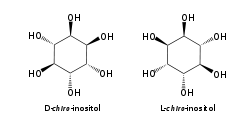
myo- scyllo- muco- chiro- 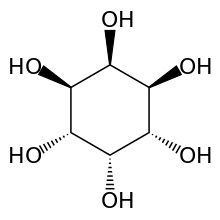
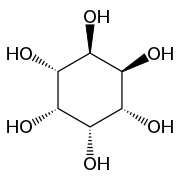
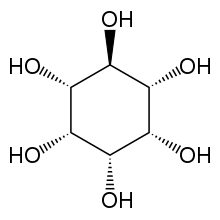
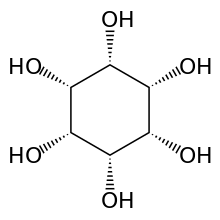
neo- allo- epi- cis-
In its most stable conformation, the myo-inositol isomer assumes the chair conformation, which moves the maximum number of hydroxyls to the equatorial position, where they are farthest apart from each other. In this conformation, the natural myo isomer has a structure in which five of the six hydroxyls (the first, third, fourth, fifth, and sixth) are equatorial, whereas the second hydroxyl group is axial.[9]
Biosynthesis
myo-Inositol is synthesized from glucose 6-phosphate (G6P) in two steps. First, G6P is isomerised by an inositol-3-phosphate synthase enzyme (for example, ISYNA1) to myo-inositol 1-phosphate, which is then dephosphorylated by an inositol monophosphatase enzyme (for example, IMPA1) to give free myo-inositol. In humans, most inositol is synthesized in the kidneys, followed by testicles, typically in amounts of a few grams per day.[2] At the peripheral level, myo-inositol is converted to D-chiro-inositol by a specific epimerase. The activity of this epimerase is insulin dependent. Worthy of note, only a small quantity of myo-inositol is converted into D-chiro-inositol and the conversion is irreversible.[10]
Inositol, phosphatidylinositol and some of their mono- and polyphosphates function as secondary messengers in a number of intracellular signal transduction pathways. They are involved in a number of biological processes, including:
- Insulin signal transduction[11]
- Cytoskeleton assembly
- Nerve guidance (epsin)
- Intracellular calcium (Ca2+) concentration control[12]
- Cell membrane potential maintenance[13]
- Breakdown of fats[14]
- Gene expression[15][16]
In one important family of pathways, phosphatidylinositol 4,5-bisphosphate (PIP2) is stored in cellular membranes until it is released by any of a number of signalling proteins and transformed into various secondary messengers, for example diacylglycerol and inositol triphosphate.[17]
Phytic acid in plants
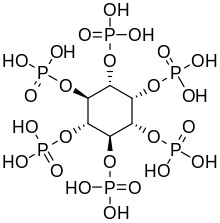
Inositol hexaphosphate, also called phytic acid or IP6, is a phytochemical and the principal storage form of phosphorus in many plant tissues, especially bran and seed.[18] Phosphorus and inositol in phytate form are not generally bioavailable to non-ruminant animals because these animals lack the digestive enzyme phytase required to remove the phosphate groups. Ruminants are readily able to digest phytate because of the phytase produced by microorganisms in the rumen.[19] Moreover, phytic acid also chelates important minerals such as calcium, magnesium, iron, and zinc, making them unabsorbable, and contributing to mineral deficiencies in people whose diets rely highly on bran and seeds for their mineral intake, such as occurs in developing countries.[20][21]
Inositol penta- (IP5), tetra- (IP4), and triphosphate (IP3) are also called "phytates".
Industrial uses
Explosives industry
At the 1936 meeting of the American Chemical Society, professor Edward Bartow of the University of Iowa presented a commercially viable means of extracting large amounts of inositol from the phytic acid naturally present in waste corn. As a possible use for the chemical, he suggested 'inositol nitrate' as a more stable alternative to nitroglycerin.[22] Today, inositol nitrate is used to gelatinize nitrocellulose in many modern explosives and solid rocket propellants.[23]
Research and clinical applications
Depression
Large doses of inositol have been studied for treatment of depression, but further study is needed to determine whether this is an effective treatment.[25]
Panic disorder and obsessive-compulsive disorder
Inositol has been found to have modest to moderate effects in patients with panic disorder or obsessive-compulsive disorder.[26][27]
Trichotillomania
High doses of inositol are sometimes used to treat trichotillomania (compulsive hair-pulling) and related disorders,[28] but a small (N=38) double-blinded placebo-controlled trial did not find a statistically significant improvement with inositol.[29]
Other illnesses
Inositol should not be routinely implemented for the management of preterm babies who have or are at a risk of infant respiratory distress syndrome (RDS).[30] Myo-inositol helps prevent neural tube defects with particular efficacy in combination with folic acid.[31]
Inositol is considered a safe and effective treatment for polycystic ovary syndrome (PCOS).[32] It works by increasing insulin sensitivity, which helps to improve ovarian function and reduce hyperandrogenism.[33] It is also shown to reduce the risk of metabolic disease in people with PCOS.[34] In addition, thanks to its role as FSH second messenger, myo-inositol is effective in restoring FSH/LH ratio and menstrual cycle regularization.[35] myo-Inositol's role as FSH second messenger leads to a correct ovarian follicle maturation and consequently to a higher oocyte quality. Improving the oocyte quality in both women with or without PCOS, myo-inositol can be considered as a possible approach for increasing the chance of success in assisted reproductive technologies.[36][37] In contrast, D-chiro-inositol can impair oocyte quality in a dose-dependent manner.[38] The high level of DCI seems to be related to elevated insulin levels retrieved in about 70% of PCOS women.[39] In this regard, insulin stimulates the irreversible conversion of myo-inositol to D-chiro-inositol causing a drastic reduction of myo-inositol. myo-Inositol depletion is particularly damaging to ovarian follicles because it is involved in FSH signaling, which is impaired due to myo-inositol depletion.[10] Recent evidence reports a faster improvement of the metabolic and hormonal parameters when these two isomers are administered in their physiological ratio. The plasmatic ratio of myo-inositol and D-chiro-inositol in healthy subjects is 40:1 of myo- and D-chiro-inositol respectively.[40] The use of the 40:1 ratio shows the same efficacy of myo-inositol alone but in a shorter time. In addition, the physiological ratio does not impair oocyte quality.[41]
The use of inositols in PCOS is gaining more importance, and an efficacy higher than 70% with a strong safety profile is reported. On the other hand, about 30% of patients could show as inositol-resistant.[42] New evidence regarding PCOS aetiopathogenesis describes an alteration in the species and the quantity of each strain characterizing the normal gastrointestinal flora. This alteration could lead to chronic, low-level inflammation and malabsorption.[43] A possible solution could be represented by the combination of myo-inositol and α-lactalbumin. This combination shows a synergic effect in increasing myo-inositol absorption.[44] A recent study reported that the myo-inositol and α-lactalbumin combination is able to increase myo-inositol plasmatic content in inositol-resistant patients with a relative improvement of hormonal and metabolic parameters.[45]
Despite its antinutrient effect, phytic acid has potential uses in endodontics, adhesive, preventive, and regenerative dentistry, and in improving the characteristics and performance of dental materials.[46]
Use as a cutting agent
Inositol has been used as an adulterant or cutting agent for many illegal drugs, such as cocaine, methamphetamine, and sometimes heroin,[47] probably because of its solubility, powdery texture, or reduced sweetness (50%) compared to more common sugars.
Inositol is also used as a stand-in film prop for cocaine in filmmaking.[48][49]
Nutritional sources
myo-Inositol is naturally present in a variety of foods, although tables of food composition do not always distinguish between lecithin, the relatively bioavailable lipid form and the biounavailable phytate/phosphate form.[5] Foods containing the highest concentrations of myo-inositol and its compounds include fruits, beans, grains, and nuts.[5] Fruits in particular, especially oranges and cantaloupe, contain the highest amounts of myo-inositol.[50] It is also present in beans, nuts, and grains, however, these contain large amounts of myo-inositol in the phytate form, which is not bioavailable without transformation by phytase enzymes. Bacillus subtilis, the microorganism which produces the fermented food natto, produces phytase enzymes that may convert phytic acid to a more bioavailable form of inositol polyphosphate in the gut.[51] Additionally, Bacteroides species in the gut secrete vesicles containing an active enzyme which converts the phytate molecule into bioavailable phosphorus and inositol polyphosphate, which is an important signaling molecule in the human body.[52]
myo-Inositol can also be found as an ingredient in energy drinks,[53] either in conjunction with or as a substitute for glucose.[54]
In humans, myo-inositol is naturally made from glucose-6-phosphate through enzymatic dephosphorylation.[50]
References
- Merck Index (11th ed.). p. 4883.
- Parthasarathy, L. K.; Seelan, R. S.; Tobias, C.; Casanova, M. F.; Parthasarathy, R. N. (2006). Mammalian inositol 3-phosphate synthase: its role in the biosynthesis of brain inositol and its clinical use as a psychoactive agent. Subcellular Biochemistry. Vol. 39. pp. 293–314. doi:10.1007/0-387-27600-9_12. ISBN 978-0-387-27599-4. PMID 17121280.
- Croze, M. L.; Soulage, C. O. (October 2013). "Potential role and therapeutic interests of myo-inositol in metabolic diseases". Biochimie. 95 (10): 1811–1827. doi:10.1016/j.biochi.2013.05.011. PMID 23764390.
- Showell, M. G.; Mackenzie-Proctor, R.; Jordan, V.; Hodgson, R.; Farquhar, C. (2018). "Inositol for subfertile women with polycystic ovary syndrome". The Cochrane Database of Systematic Reviews. 2018 (12): CD012378. doi:10.1002/14651858.CD012378.pub2. PMC 6516980. PMID 30570133.
- Clements, R. S.; Darnell, B. (September 1980). "myo-Inositol content of common foods: development of a high-myo-inositol diet". The American Journal of Clinical Nutrition. 33 (9): 1954–1967. doi:10.1093/ajcn/33.9.1954. PMID 7416064. S2CID 4442333.
- "Phytic acid". www.phytochemicals.info. Archived from the original on 2017-08-06. Retrieved 2017-10-02.
- Reynolds, J. E. F. (1993). Martindale: The Extra Pharmacopoeia. Vol. 30. Pennsylvania: Rittenhouse Book Distributors. p. 1379. ISBN 978-0-85369-300-0.
An isomer of glucose that has traditionally been considered to be a B vitamin although it has an uncertain status as a vitamin has not been identified in man
- Majumder, A. L.; Biswas, B. B. (2006-10-03). Biology of Inositols and Phosphoinositides. Springer Science & Business Media. ISBN 9780387276007.
- Brady, S.; Siegel, G.; Albers, R. W.; Price, D. (2005). Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Academic Press. p. 348. ISBN 9780080472072.
- Carlomagno, G.; Unfer, V.; Roseff, S. (2011). "The D-chiro-inositol paradox in the ovary". Fertility and Sterility. 95 (8): 2515–6. doi:10.1016/j.fertnstert.2011.05.027. PMID 21641593.
- Larner, J. (2002). "D-chiro-Inositol—its functional role in insulin action and its deficit in insulin resistance". International Journal of Experimental Diabetes Research. 3 (1): 47–60. doi:10.1080/15604280212528. PMC 2478565. PMID 11900279.
- Gerasimenko, J. V.; Flowerdew, S. E.; Voronina, S. G.; Sukhomlin, T. K.; Tepikin, A. V.; Petersen, O. H.; Gerasimenko, O. V. (December 2006). "Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors". The Journal of Biological Chemistry. 281 (52): 40154–40163. doi:10.1074/jbc.M606402200. PMID 17074764.
- Kukuljan, M.; Vergara, L.; Stojilković, S. S. (February 1997). "Modulation of the kinetics of inositol 1,4,5-trisphosphate-induced [Ca2+]i oscillations by calcium entry in pituitary gonadotrophs". Biophysical Journal. 72 (2 Pt 1): 698–707. Bibcode:1997BpJ....72..698K. doi:10.1016/S0006-3495(97)78706-X. PMC 1185595. PMID 9017197.
- Rapiejko, P. J.; Northup, J. K.; Evans, T.; Brown, J. E.; Malbon, C. C. (November 1986). "G-proteins of fat-cells. Role in hormonal regulation of intracellular inositol 1,4,5-trisphosphate". The Biochemical Journal. 240 (1): 35–40. doi:10.1042/bj2400035. PMC 1147372. PMID 3103610.
- Shen, X.; Xiao, H.; Ranallo, R.; Wu, W.-H.; Wu, C. (January 2003). "Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates". Science. 299 (5603): 112–114. Bibcode:2003Sci...299..112S. doi:10.1126/science.1078068. PMID 12434013. S2CID 8381889.
- Steger, D. J.; Haswell, E. S.; Miller, A. L.; Wente, S. R.; O'Shea, E. K. (January 2003). "Regulation of chromatin remodeling by inositol polyphosphates". Science. 299 (5603): 114–116. Bibcode:2003Sci...299..114S. doi:10.1126/science.1078062. PMC 1458531. PMID 12434012.
- Mathews, C. K.; Van Holde, K. E.; Ahern, K. G. (2000). Biochemistry (3rd ed.). San Francisco, CA: Benjamin Cummings. p. 855. ISBN 978-0805330663. OCLC 42290721.
- "Phytic acid". www.phytochemicals.info. Archived from the original on 7 March 2018. Retrieved 2018-05-02.
- Klopfenstein, T. J.; Angel, R.; Cromwell, G.; Erickson, G. E.; Fox, D. G.; Parsons, C.; Satter, L. D.; Sutton, A. L.; Baker, D. H. (July 2002). "Animal diet modification to decrease the potential for nitrogen and phosphorus pollution". Council for Agricultural Science and Technology. 21. Archived from the original on 2011-06-11.
- Hurrell, R. F. (September 2003). "Influence of vegetable protein sources on trace element and mineral bioavailability". The Journal of Nutrition. 133 (9): 2973S–2977S. doi:10.1093/jn/133.9.2973S. PMID 12949395.
- Committee on Food Protection; Food and Nutrition Board; National Research Council (1973). "Phytates". Toxicants Occurring Naturally in Foods. National Academy of Sciences. pp. 363–371. ISBN 978-0-309-02117-3.
- Laurence, W. L. (April 17, 1936). "Corn by-product yields explosive". The New York Times. p. 7. Archived from the original on 2013-05-12.
- Ledgard, J. (2007). The Preparatory Manual of Explosives. Ledgard. p. 366. ISBN 9780615142906.
- Chatterjee, J.; Majumder, A. L. (September 2010). "Salt-induced abnormalities on root tip mitotic cells of Allium cepa: prevention by inositol pretreatment". Protoplasma. 245 (1–4): 165–172. doi:10.1007/s00709-010-0170-4. PMID 20559853. S2CID 9128286.
- Taylor, M. J.; Wilder, H.; Bhagwagar, Z.; Geddes, J. (2004). "Inositol for depressive disorders". The Cochrane Database of Systematic Reviews. 2004 (2): CD004049. doi:10.1002/14651858.CD004049.pub2. PMC 6984679. PMID 15106232.
- Benjamin, J. (July 1995). "Double-blind, placebo-controlled, crossover trial of inositol treatment for panic disorder". Am J Psychiatry. 152 (7): 1084–6. doi:10.1176/ajp.152.7.1084. PMID 7793450.
- Fux, J. (1996). "Inositol treatment of obsessive-compulsive disorder". Am J Psychiatry. 153 (9): 1219–21. doi:10.1176/ajp.153.9.1219. PMID 8780431.
- Sani, Gabriele; Gualtieri, Ida; Paolini, Marco; Bonanni, Luca; Spinazzola, Edoardo; Maggiora, Matteo; Pinzone, Vito; Brugnoli, Roberto; Angeletti, Gloria; Girardi, Paolo; Rapinesi, Chiara; Kotzalidis, Georgios D. (25 July 2019). "Drug Treatment of Trichotillomania (Hair-Pulling Disorder), Excoriation (Skin-picking) Disorder, and Nail-biting (Onychophagia)". Current Neuropharmacology. 17 (8): 775–786. doi:10.2174/1570159X17666190320164223. PMC 7059154. PMID 30892151.
- Leppink, Eric W.; Redden, Sarah A.; Grant, Jon E. (March 2017). "A double-blind, placebo-controlled study of inositol in trichotillomania". International Clinical Psychopharmacology. 32 (2): 107–114. doi:10.1097/YIC.0000000000000156. PMID 27824633. S2CID 3273619.
- Howlett, Alexandra; Ohlsson, Arne; Plakkal, Nishad (8 July 2019). "Inositol in preterm infants at risk for or having respiratory distress syndrome". The Cochrane Database of Systematic Reviews. 7 (7): CD000366. doi:10.1002/14651858.CD000366.pub4. ISSN 1469-493X. PMC 6613728. PMID 31283839.
- Cavalli, P.; Ronda, E. (2017). "Myoinositol: the bridge (PONTI) to reach a healthy pregnancy". International Journal of Endocrinology. 2017: 5846286. doi:10.1155/2017/5846286. PMC 5274721. PMID 28243254.
- Greff, Dorina; Juhász, Anna E.; Váncsa, Szilárd; Váradi, Alex; Sipos, Zoltán; Szinte, Julia; Park, Sunjune; Hegyi, Péter; Nyirády, Péter; Ács, Nándor; Várbíró, Szabolcs; Horváth, Eszter M. (2023). "Inositol is an effective and safe treatment in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials". Reproductive Biology and Endocrinology. 21 (1): 10. doi:10.1186/s12958-023-01055-z. PMC 9878965. PMID 36703143.
- Monastra, G.; Unfer, V.; Harrath, A. H.; Bizzarri, M. (January 2017). "Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients". Gynecological Endocrinology. 33 (1): 1–9. doi:10.1080/09513590.2016.1247797. hdl:11573/944617. PMID 27898267. S2CID 24836559.
- Nordio, M.; Proietti, E. (May 2012). "The combined therapy with myo-inositol and D-chiro-inositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo-inositol supplementation alone". European Review for Medical and Pharmacological Sciences. 16 (5): 575–581. PMID 22774396.
- Unfer, V.; et al. (2012). "Effects of myo-inositol in women with PCOS: a systematic review of randomized controlled trials". Gynecological Endocrinology. 28 (7): 509–15. doi:10.3109/09513590.2011.650660. PMID 22296306. S2CID 24582338.
- Ciotta, L.; et al. (2011). "Effects of myo-inositol supplementation on oocyte's quality in PCOS patients: a double blind trial". European Review for Medical and Pharmacological Sciences. 15 (5): 509–14. PMID 21744744.
- Papaleo, E.; et al. (2009). "Contribution of myo-inositol to reproduction". European Journal of Obstetrics & Gynecology and Reproductive Biology. 147 (2): 120–3. doi:10.1016/j.ejogrb.2009.09.008. PMID 19800728.
- Isabella, R.; Raffone, E. (2012). "Does ovary need D-chiro-inositol?". Journal of Ovarian Research. 5 (1): 14. doi:10.1186/1757-2215-5-14. PMC 3447676. PMID 22587479.
- Moghetti, P. (2016). "Insulin resistance and polycystic ovary syndrome". Current Pharmaceutical Design. 22 (36): 5526–5534. doi:10.2174/1381612822666160720155855. PMID 27510482.
- Facchinetti, F.; et al. (2015). "Results from the International Consensus Conference on myo-Inositol and D-chiro-Inositol in Obstetrics and Gynecology: the link between metabolic syndrome and PCOS". European Journal of Obstetrics & Gynecology and Reproductive Biology. 195: 72–6. doi:10.1016/j.ejogrb.2015.09.024. PMID 26479434.
- Colazingari, S.; et al. (2013). "The combined therapy myo-inositol plus D-chiro-inositol, rather than D-chiro-inositol, is able to improve IVF outcomes: results from a randomized controlled trial". Archives of Gynecology and Obstetrics. 288 (6): 1405–11. doi:10.1007/s00404-013-2855-3. PMID 23708322. S2CID 45611717.
- Kamenov, Z.; et al. (2015). "Ovulation induction with myo-inositol alone and in combination with clomiphene citrate in polycystic ovarian syndrome patients with insulin resistance". Gynecological Endocrinology. 31 (2): 131–5. doi:10.3109/09513590.2014.964640. PMID 25259724. S2CID 24469378.
- González, F. (2012). "Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction". Steroids. 77 (4): 300–5. doi:10.1016/j.steroids.2011.12.003. PMC 3309040. PMID 22178787.
- Monastra, G.; et al. (2018). "alpha-Lactalbumin effect on myo-inositol intestinal absorption: in vivo and in vitro". Current Drug Delivery. 15 (9): 1305–1311. doi:10.2174/1567201815666180509102641. PMID 29745333. S2CID 13691602.
- Oliva, M. M.; et al. (2018). "Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women". Journal of Ovarian Research. 11 (1): 38. doi:10.1186/s13048-018-0411-2. PMC 5944130. PMID 29747700.
- Nassar M, Nassar R, Maki H, Al-Yagoob A, Hachim M, Senok A, Williams D, Hiraishi N (March 2021). "Phytic Acid: Properties and Potential Applications in Dentistry". Frontiers in Materials. 8: 29. Bibcode:2021FrMat...8...29N. doi:10.3389/fmats.2021.638909.
- "Inositol, Nerve guidance, Cutting agent manufacturer". Tianyu Feed Additive. Archived from the original on 2014-09-08. Retrieved 2013-07-21.
- Golianopoulos, T. (2012-05-12). "Drug doubles: What actors actually toke, smoke and snort on camera". Wired. Archived from the original on 2012-05-14. Retrieved 2012-05-14.
- How Fake Drugs Are Made For Movies | Movies Insider, retrieved 2022-09-26
- Awuchi, Chinaza (2017). "Sugar Alcohols: Chemistry, Production, Health Concerns and Nutritional Importance of Mannitol, Sorbitol, Xylitol, and Erythritol". International Journal of Advanced Academic Research. 5 (11): 1954–1967.
- Borgi MA, Boudebbouze S, Mkaouar H, Maguin E, Rhimi M (2015). "Bacillus phytases: Current status and future prospects". Bioengineered. 5 (4): 233–236. doi:10.1080/21655979.2015.1048050. PMC 4601277. PMID 25946551.
- Stentz R, Osborne S, Horn N, Li AW, Hautefort I, Bongaerts R, Rouyer M, Bailey P, Shears SB, Hemmings AM, Brearley CA, Carding SR (27 February 2014). "A Bacterial Homolog of a Eukaryotic Inositol Phosphate Signaling Enzyme Mediates Cross-kingdom Dialog in the Mammalian Gut". Cell Reports. 6 (4): 646–656. doi:10.1016/j.celrep.2014.01.021. PMC 3969271. PMID 24529702.
- Ehlers, Anke; Marakis, Georgios; Lampen, Alfonso; Hirsch-Ernst, Karen Ildico (2019-08-01). "Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe". Food and Chemical Toxicology. 130: 109–121. doi:10.1016/j.fct.2019.05.028. ISSN 0278-6915. PMID 31112702.
- DiSalvo, David. "We Know About Caffeine in Energy Drinks Like Monster, But What About the Other Ingredients?". Forbes. Retrieved 2020-12-22.
External links
- U.S. National Library of Medicine: Drug Information Portal – Inositol
- Inositol MS Spectrum
- Inositol bound to proteins in the PDB
