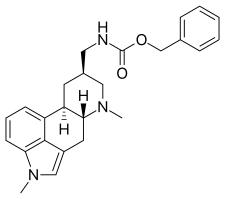
Metergoline
Metergoline (INNTooltip INN, BANTooltip British Approved Name), also known as methergoline and sold under the brand names Contralac (veterinary) and Liserdol (clinical), is a monoaminergic medication of the ergoline group which is used as a prolactin inhibitor in the treatment of hyperprolactinemia (high prolactin levels) and to suppress lactation.[1][2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Contralac, Liserdol |
| Other names | Methergoline; FI-6337; [(8β)-1,6-Dimethylergolin-8-yl)methyl]carbamic acid phenylmethyl ester |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.037.881 |
| Chemical and physical data | |
| Formula | C25H29N3O2 |
| Molar mass | 403.526 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pharmacology
Pharmacodynamics
Metergoline is a ligand of various serotonin and dopamine receptors.[4][5][6][7][8]
| Site | Affinity (Ki [nM]) | Efficacy (Emax [%]) | Action |
|---|---|---|---|
| 5-HT1A | 4.3 | ? | Agonist |
| 5-HT1B | 5.2–36 | ? | Partial agonist |
| 5-HT1D | 0.60–11.7 | ? | Partial agonist |
| 5-HT1E | 776–1,122 | ? | ? |
| 5-HT1F | 339–341 | ? | ? |
| 5-HT2A | 0.12–2.3 | ? | Antagonist |
| 5-HT2B | 0.71–1.8 | ? | Antagonist |
| 5-HT2C | 0.18–1.8 | ? | Antagonist |
| 5-HT3 | >5,000–7,400 | ? | ? |
| 5-HT4 | 354 | ? | ? |
| 5-HT5A | 630 | ? | ? |
| 5-HT5B | 1,000 | ? | ? |
| 5-HT6 | 61–400 | ? | ? |
| 5-HT7 | 6.4–6.5 | ? | Antagonist |
| D2 | ? | ? | Agonist |
| Notes: All sites are human except 5-HT3 (rat/pig), 5-HT4 (pig), and 5-HT5B (rat—no human counterpart).[4] | |||
References
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 661–. ISBN 978-3-88763-075-1.
- Donald C. Plumb (21 February 2018). Plumb's Veterinary Drug Handbook: Pocket. John Wiley & Sons. pp. 1057–. ISBN 978-1-119-34649-4.
- Richard W. Nelson; C. Guillermo Couto (2 December 2008). Small Animal Internal Medicine - E-Book. Elsevier Health Sciences. pp. 927–. ISBN 978-0-323-06512-2.
- "PDSP Database - UNC". pdsp.unc.edu. Archived from the original on 16 April 2021. Retrieved 15 January 2022.
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP (June 1994). "International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin)". Pharmacol Rev. 46 (2): 157–203. PMID 7938165.
- Hamon M, Mallat M, Herbet A, et al. (February 1981). "[3H]Metergoline: a new ligand of serotonin receptors in the rat brain". Journal of Neurochemistry. 36 (2): 613–26. doi:10.1111/j.1471-4159.1981.tb01634.x. PMID 7463079. S2CID 20259621.
- Miller KJ, King A, Demchyshyn L, Niznik H, Teitler M (1992). "Agonist activity of sumatriptan and metergoline at the human 5-HT1D beta receptor: further evidence for a role of the 5-HT1D receptor in the action of sumatriptan". Eur. J. Pharmacol. 227 (1): 99–102. doi:10.1016/0922-4106(92)90149-P. PMID 1330643.
- Webster J (December 1999). "Dopamine agonist therapy in hyperprolactinemia". J Reprod Med. 44 (12 Suppl): 1105–10. PMID 10649819.
- Pertz H, Eich E (1999). "Ergot Alkaloids and their Derivatives as Ligands for Serotoninergic, Dopaminergic, and Adrenergic Receptors" (PDF). Ergot. pp. 432–462. doi:10.1201/9780203304198-21. ISBN 9780429219764. Archived from the original (PDF) on 2021-04-16.
- Pauwels PJ (September 1997). "5-HT 1B/D receptor antagonists". Gen Pharmacol. 29 (3): 293–303. doi:10.1016/s0306-3623(96)00460-0. PMID 9378233.
- Hutcheson JD, Setola V, Roth BL, Merryman WD (November 2011). "Serotonin receptors and heart valve disease--it was meant 2B". Pharmacol Ther. 132 (2): 146–57. doi:10.1016/j.pharmthera.2011.03.008. PMC 3179857. PMID 21440001.
External links
- Metergoline at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.