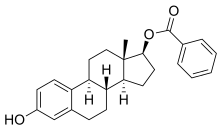
Estradiol 17β-benzoate
 | |
| Clinical data | |
|---|---|
| Other names | E2-17B; ZYC30 |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H28O3 |
| Molar mass | 376.496 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Estradiol 17β-benzoate (E2-17B) is an estrogen and an estrogen ester—specifically, the C17β benzoate ester of estradiol—which was never marketed.[1][2][3] It is the C17β positional isomer of the better-known and clinically used estradiol ester estradiol benzoate (estradiol 3-benzoate; Progynon-B).[1] Estradiol 17β-benzoate was first described in the 1930s.[4]
See also
References
- 1 2 Junkmann K, Witzel H (1957). "Chemie und Pharmakologie von Steroidhormon-Estern" [Chemistry and pharmacology of steroid hormone esters]. Z Vitam Horm Fermentforsch (in German). 9 (1–2): 97–143 contd. PMID 13531579.
- ↑ Perez, Evelyn; Cai, Zu Yun; Covey, Douglas F.; Simpkins, James W. (2005). "Neuroprotective effects of estratriene analogs: structure-activity relationships and molecular optimization". Drug Development Research. 66 (2): 78–92. doi:10.1002/ddr.20047. ISSN 0272-4391. S2CID 86133327.
- ↑ Yi KD, Perez E, Yang S, Liu R, Covey DF, Simpkins JW (March 2011). "The assessment of non-feminizing estrogens for use in neuroprotection". Brain Res. 1379: 61–70. doi:10.1016/j.brainres.2010.11.058. PMC 3048764. PMID 21111714.
- ↑ Parkes AS (April 1937). "Relative duration of action of various esters of oestrone, oestradiol and oestriol". Biochem. J. 31 (4): 579–85. doi:10.1042/bj0310579. PMC 1266977. PMID 16746375.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.