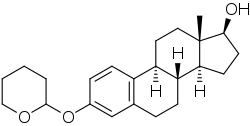
Estradiol 3-tetrahydropyranyl ether
 | |
| Clinical data | |
|---|---|
| Other names | NSC-86473; Estra-1,3,5(10)-triene-17β-diol 3-(tetrahydropyran-2-yl) ether; 3-(Tetrahydro-2H-pyran-2-yloxy)estra-1,3,5(10)-trien-3-ol |
| Routes of administration | By mouth[1][2][3][4] |
| Drug class | Estrogen; Estrogen ether |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H32O3 |
| Molar mass | 356.506 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Estradiol 3-tetrahydropyranyl ether is a synthetic estrogen and estrogen ether which was never marketed.[1][3][4][2][5][6][7] It has been reported to possess improved oral activity relative to estradiol.[1][3][4] One study in animals found that it had 15 times the oral activity of estradiol.[1][3]
See also
References
- 1 2 3 4 Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1395–. ISBN 978-1-60913-345-0.
- 1 2 Locardi G (February 1972). "[Clinical pharmacology and biological characteristics of 3-tetrahydropyranyl ether of 17-beta-estradiol]". Minerva Ginecol (in Italian). 24 (2): 70–81. PMID 4603402.
- 1 2 3 4 Cross, A.D.; Harrison, I.T.; Kincl, F.A.; Farkas, E.; Kraay, R.; Dorfman, R.I. (1964). "Steroids CCLXX. Biologically-active labile ethers II. A new group of potent orally-active estrogens". Steroids. 4 (3): 423–432. doi:10.1016/0039-128X(64)90155-2. ISSN 0039-128X.
- 1 2 3 Kincl FA, Dorfman RI (August 1965). "Antifertility activity of various steroids in the female rat" (PDF). J. Reprod. Fertil. 10: 105–13. doi:10.1530/jrf.0.0100105. PMID 14337800.
- ↑ Plancher G (September 1971). "[On some pharmacobiological characteristics of a new estrogen derivative (3-tetrahydropyranyl ether of 17-beta-estradiol)]". Minerva Ginecol (in Italian). 23 (17): 671–8. PMID 5131894.
- ↑ Andreoli C, Lenzi G (September 1971). "[Clinical pharmacological aspects of a new hormone derivative (3-tetrahydropyranyl ether of 17-beta-estradiol)]". Minerva Ginecol (in Italian). 23 (18): 711–24. PMID 5131179.
- ↑ Boucheau V, Renaud M, Rolland de Ravel M, Mappus E, Cuilleron CY (May 1990). "Proton and carbon-13 nuclear magnetic resonance spectroscopy of diastereoisomeric 3- and 17 beta-tetrahydropyranyl ether derivatives of estrone and estradiol". Steroids. 55 (5): 209–21. doi:10.1016/0039-128X(90)90018-7. PMID 2163125. S2CID 1476967.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.