Foscarnet
 | |
 | |
| Names | |
|---|---|
| Trade names | Foscavir |
| Other names | Foscarnet sodium, phosphonomethanoic acid, dihydroxyphosphinecarboxylic acid oxide |
IUPAC name
| |
| Clinical data | |
| Main uses | Cytomegalovirus (CMV), herpes simplex virus (HSV), varicella zoster virus (VZV)[1] |
| Side effects | Fever, nausea, low red blood cells, kidney problems, seizures, headache[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601144 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | NA |
| Protein binding | 14–17% |
| Elimination half-life | 3.3–6.8 hours |
| Chemical and physical data | |
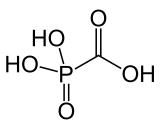
| Formula | CH3O5P |
| Molar mass | 126.004 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Foscarnet, sold under the brand name Foscavir, is an antiviral used to treat cytomegalovirus (CMV), herpes simplex virus (HSV), and varicella zoster virus (VZV).[1] Primarily this is those who are immunocompromised such as due to HIV/AIDS.[1] It is given by injection into a vein.[3]
Common side effects include fever, nausea, low red blood cells, kidney problems, seizures, and headache.[1] Other side effects may include electrolyte abnormalities, prolonged QT, and anaphylaxis.[1] Safety in pregnancy is unclear.[1] It acts similar to pyrophosphate as a viral DNA polymerase inhibitor.[1]
Foscarnet was approved for medical use in the United States in 1991.[4][1] In the United Kingdom 6 grams costs the NHS about £120 as of 2021.[3] This amount in the United States cost about 4,800 USD.[5]
Medical use
It is used to treat herpes viruses, including drug-resistant cytomegalovirus (CMV) and herpes simplex viruses types 1 and 2 (HSV-1 and HSV-2). It is particularly used to treat CMV retinitis. Foscarnet can be used to treat highly treatment-experienced patients with HIV as part of salvage therapy.[6][7][8]
Dosage
Foscarnet is administered by intravenous infusion or intravitreous injection.
It is given at a dose of 60 mg/kg three times per day for CMV and 40 mg/kg three times per day for HSV.[3]
Side effects
- Nephrotoxicity — increase in serum creatinine levels occurs on average in 45% of patients receiving foscarnet. Other nephrotoxic drugs should be avoided. Nephrotoxicity is usually reversible and can be reduced by dosage adjustment and adequate hydration.
- Electrolyte disturbances — changes in calcium, magnesium (Harisson 16th ed page2244) potassium and phosphate levels occurs commonly and regular monitoring of electrolytes is necessary to avoid clinical toxicity.
- Genital ulceration — occurs more commonly in men and usually occurs during induction use of foscarnet. It is most likely a contact dermatitis due to high concentrations of foscarnet in urine. It usually resolves rapidly following discontinuation of the drug.
- CNS — paresthesia, irritability and hallucinations.
Mechanism of action
Foscarnet is a structural mimic of the anion pyrophosphate that selectively inhibits the pyrophosphate binding site on viral DNA polymerases at concentrations that do not affect human DNA polymerases.[9]
In individuals treated with the DNA polymerase inhibitors acyclovir or ganciclovir, HSV or CMV particles can develop mutant protein kinases (thymidine kinase or UL97 protein kinase, respectively) that make them resistant to these antiviral drugs. However, unlike acyclovir and ganciclovir, foscarnet is not activated by viral protein kinases, making it useful in acyclovir- or ganciclovir-resistant HSV and CMV infections.
However, acyclovir- or ganciclovir-resistant mutants with alterations in viral DNA polymerase may also be resistant to foscarnet.[10][11]
Chemistry
Foscarnet is the conjugate base of a chemical compound with the formula HO2CPO3H2.
References
- 1 2 3 4 5 6 7 8 9 "Foscarnet Monograph for Professionals". Drugs.com. Archived from the original on 15 November 2021. Retrieved 13 December 2021.
- ↑ "Foscavir- foscarnet sodium injection, solution". DailyMed. 23 April 2020. Archived from the original on 26 March 2021. Retrieved 6 November 2020.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 678. ISBN 978-0857114105.
- ↑ Long, Sarah S.; Pickering, Larry K.; Prober, Charles G. (2012). Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. p. 1502. ISBN 978-1437727029. Archived from the original on 2019-12-29. Retrieved 2021-04-29.
- ↑ "Foscarnet Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 21 April 2021. Retrieved 13 December 2021.
- ↑ Canestri A, Ghosn J, Wirden M, et al. (2006). "Foscarnet salvage therapy for patients with late-stage HIV disease and multiple drug resistance". Antivir. Ther. (Lond.). 11 (5): 561–6. PMID 16964823.
- ↑ Mathiesen S, Dam E, Roge B, et al. (2007). "Long-term foscarnet therapy remodels thymidine analogue mutations and alters resistance to zidovudine and lamivudine in HIV-1". Antivir. Ther. (Lond.). 12 (3): 335–43. PMID 17591023.
- ↑ Meyer PR, Rutvisuttinunt W, Matsuura SE, So AG, Scott WA (2007). "Stable complexes formed by HIV-1 reverse transcriptase at distinct positions on the primer-template controlled by binding deoxynucleoside triphosphates or foscarnet". J. Mol. Biol. 369 (1): 41–54. doi:10.1016/j.jmb.2007.03.006. PMC 1986715. PMID 17400246.
- ↑ Meyer PR, Rutvisuttinunt W, Matsuura SE, So AG, Scott WA (May 2007). "Stable complexes formed by HIV-1 reverse transcriptase at distinct positions on the primer-template controlled by binding deoxynucleoside triphosphates or foscarnet". J. Mol. Biol. 369 (1): 41–54. doi:10.1016/j.jmb.2007.03.006. PMC 1986715. PMID 17400246.
- ↑ Bonnafous P, Naesens L, Petrella S, et al. (2007). "Different mutations in the HHV-6 DNA polymerase gene accounting for resistance to foscarnet". Antivir. Ther. (Lond.). 12 (6): 877–88. PMID 17926642.
- ↑ Tchesnokov EP, Gilbert C, Boivin G, Götte M (February 2006). "Role of helix P of the human cytomegalovirus DNA polymerase in resistance and hypersusceptibility to the antiviral drug foscarnet". J. Virol. 80 (3): 1440–50. doi:10.1128/JVI.80.3.1440-1450.2006. PMC 1346920. PMID 16415021.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Foscarnet sodium". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-05-03. Retrieved 2021-04-29.