Tildipirosin
 | |
| Clinical data | |
|---|---|
| Trade names | Zuprevo |
| License data |
|
| Routes of administration | Intramuscular, subcutaneous |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Identifiers | |
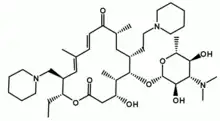
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.168.011 |
| Chemical and physical data | |
| Formula | C41H71N3O8 |
| Molar mass | 734.032 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Tildipirosin, sold under the brand name Zuprevo is an antibiotic used in pigs and cattle.[1][3]
Medical uses
In the United States, tildipirosin is indicated for the treatment or control of bovine respiratory disease associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in beef and non-lactating dairy cattle.[1]
In the European Union, tildipirosin is indicated for the treatment and metaphylaxis of swine respiratory disease associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica, and Haemophilus parasuis sensitive to tildipirosin;[3] and for the treatment and prevention of bovine respiratory disease associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni sensitive to tildipirosin.[3]
References
- 1 2 3 "Zuprevo- tildipirosin injection, solution". DailyMed. U.S. National Library of Medicine. 11 January 2013. Retrieved 21 August 2023.
- ↑ Budde JA, McCluskey DM (2023). Plumb's Veterinary Drug Handbook (Tenth ed.). Wiley. p. 1235. ISBN 978-1-394-17220-7.
- 1 2 3 4 "Zuprevo EPAR". European Medicines Agency. 3 February 2022. Retrieved 21 August 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.