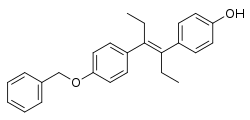
Diethylstilbestrol monobenzyl ether
 | |
| Clinical data | |
|---|---|
| Trade names | Monozol, Hypantin, Pituitrope |
| Other names | Benzelstilbestrol |
| Drug class | Nonsteroidal estrogen; Estrogen ether |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C25H26O2 |
| Molar mass | 358.481 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Diethylstilbestrol monobenzyl ether (brand names Monozol, Hypantin, Pituitrope), also known as benzelstilbestrol, is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ether of diethylstilbestrol (DES) that is described as a pituitary gland inhibitor (antigonadotropin) and was formerly marketed but is now no longer available.[1][2] It was first synthesized by Wallace & Tiernan Company in 1952, and was described by them as having only weak estrogenic activity.[3] The drug was used to treat gynecological conditions and infertility in women.[2]
See also
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 397. ISBN 978-1-4757-2085-3.
- 1 2 Marsh M, Ronner W (29 December 2010). The Fertility Doctor: John Rock and the Reproductive Revolution. JHU Press. pp. 144–. ISBN 978-1-4214-0208-6.
- ↑ Harrison RG (1957). Studies on Fertility. Blackwell. pp. 135–136.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.