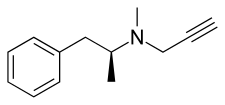
D-Deprenyl
d-Deprenyl, also known as or dextro-N-propargyl-N-methylamphetamine, is an MAO-B inhibitor that metabolizes into d-amphetamine and d-methamphetamine and is therefore also a norepinephrine–dopamine releasing agent.[1][2][3][4][5] It is the opposite enantiomer of l-deprenyl (selegiline).
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H17N |
| Molar mass | 187.286 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
l-Deprenyl, also an MAO-B inhibitor, metabolizes to l-amphetamine and l-methamphetamine, which are both norepinephrine releasing agents. In contrast, d-deprenyl additionally has dopaminergic effects and has been found to be reinforcing in scientific research, whereas l-deprenyl is not known to have any appreciable psychological reinforcement.[6][7]
In addition to its actions as an MAO-B inhibitor and NDRA, d-deprenyl has been found to bind with high affinity to the σ1 receptor (Ki = 79 nM) similarly to various other amphetamine derivatives.[8][9] Its l-isomer, selegiline, binds with 3.5-fold lower affinity in comparison.[8][9]
See also
References
- C Thiffault; R Quirion; J Poirier (October 1997). "The effect of l-deprenyl, d-deprenyl and MDL72974 on mitochondrial respiration: a possible mechanism leading to an adaptive increase in superoxide dismutase activity". Molecular Brain Research. 49 (1–2): 127–136. doi:10.1016/S0169-328X(97)00135-6. PMID 9387872.
- Srinivasan ThyagaRajan; Kelley S. Madden; Gary W. Boehm; Suzanne Y. Stevens; David L. Felten; Denise L. Bellinger (January 2013). "l-Deprenyl Reverses Age-Associated Decline in Splenic Norepinephrine, Interleukin-2 and Interferon-γ Production in Old Female F344 Rats". Neuroimmunomodulation. 20 (2): 72–78. doi:10.1159/000345043. PMC 3695399. PMID 23207416.
- Dhanasekharan Muralikrishnan; Supriti Samantaray; Kochupurackal P. Mohanakumar (October 2003). "D-deprenyl protects nigrostriatal neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity". Synapse. 50 (1): 7–13. doi:10.1002/syn.10239. PMID 12872288.
- László Simon; Géza Szilágyi; Zoltán Bori; Péter Orbay; Zoltán Nagy (November 2001). "(−)-d-Deprenyl attenuates apoptosis in experimental brain ischaemia". European Journal of Pharmacology. 430 (2–3): 235–241. doi:10.1016/S0014-2999(01)01375-9. PMID 11711036.
- S Yasar; C W Schindler; E B Thorndike; I Szelenyi; S R Goldberg (April 1993). "Evaluation of the stereoisomers of deprenyl for amphetamine-like discriminative stimulus effects in rats". Journal of Pharmacology and Experimental Therapeutics. 265 (1): 1–6. PMID 8473997.
- Yasar S; Gaál J; Panlilio LV; et al. (January 2006). "A comparison of drug-seeking behavior maintained by d-amphetamine, l-deprenyl (selegiline) and d-deprenyl under a second-order schedule in squirrel monkeys". Psychopharmacology. 183 (4): 413–21. doi:10.1007/s00213-005-0200-7. PMC 1360227. PMID 16292593.
- Winger GD, Yasar S, Negus SS, Goldberg SR (December 1994). "Intravenous self-administration studies with l-deprenyl (selegiline) in monkeys" (PDF). Clinical Pharmacology & Therapeutics. 56 (6): 774–780. doi:10.1038/clpt.1994.208. hdl:2027.42/110034. PMID 7995020. S2CID 10021258.
- Yossef Itzhak (1994). Sigma Receptors. Academic Press. p. 84. ISBN 978-0-12-376350-1.
- T. W. Stone (January 1993). Acetylcholine, Sigma Receptors, CCK and Eicosanoids, Neurotoxins. Taylor & Francis. pp. 124–. ISBN 978-0-7484-0063-8.