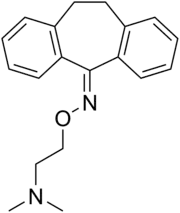
Noxiptiline
Noxiptiline (brand names Agedal, Elronon, Nogedal), also known as noxiptyline and dibenzoxine, is a tricyclic antidepressant (TCA) that was introduced in Europe in the 1970s for the treatment of depression.[2][3][4] It has imipramine-like effects,[5] acting as a serotonin and norepinephrine reuptake inhibitor, among other properties.[6][7] Of the TCAs, noxiptiline has been described as one of the most effective, rivaling amitriptyline in clinical efficacy.[8][9]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Agedal, Elronon, Nogedal |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H22N2O |
| Molar mass | 294.398 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
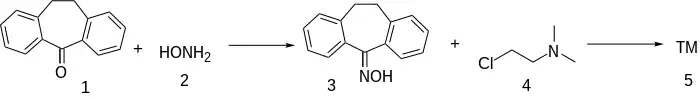
Synthesis
Ths synthesis is similar to that for Demexiptiline.
The condensation of dibenzosuberone (1) with hydroxylamine (2) gives the corresponding oxime [1785-74-6] (3). This is then reacted with 2-(dimethylamine)ethyl chloride [4584-46-7] (4).
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. p. 753. ISBN 3-88763-075-0.
- Aronson, Jeffrey Kenneth (2008). Meyler's Side Effects of Psychiatric Drugs (Meylers Side Effects). Amsterdam: Elsevier Science. p. 34. ISBN 978-0-444-53266-4.
- Dictionary of organic compounds. London: Chapman & Hall. 1996. p. 4868. ISBN 0-412-54090-8.
- Mutschler, Ernst (1995). Drug actions: basic principles and therapeutic aspects. Stuttgart, Germany: Medpharm Scientific Pub. p. 127. ISBN 0-8493-7774-9. Retrieved January 30, 2013.
- Ernst Jucker; S. Ren; Soudijn, W.; Wijngaarden, I. van; M. Kumari; D. Poyner; M. Bushfield; H. Horikoshi; Fujiwara, Tamenari (2000). Progress in Drug Research, Volume 54 (Progress in Drug Research). Boston: Birkhauser. p. 81. ISBN 3-7643-6113-1.
- Barth N, Manns M, Muscholl E (1975). "Arrhythmias and inhibition of noradrenaline uptake caused by tricyclic antidepressants and chlorpromazine on the isolated perfused rabbit heart". Naunyn-Schmiedeberg's Archives of Pharmacology. 288 (2–3): 215–31. doi:10.1007/BF00500528. PMID 1161046. S2CID 11641400.
- Beresewicz M, Bidzińska E, Koszewska I, Puzyński S (1991). "[Results of using tricyclic antidepressive drugs in the treatment of endogenous depression (comparative analysis of 7 drugs)]". Psychiatria Polska (in Polish). 25 (3–4): 13–8. PMID 1687987.
- Lingjaerde O, Asker T, Bugge A, et al. (January 1975). "Noxiptilin (Agedal)--a new tricyclic antidepressant with a faster onset of action? A double-blind, multicentre comparison with amitriptyline". Pharmakopsychiatrie, Neuro-Psychopharmakologie. 8 (1): 26–35. doi:10.1055/s-0028-1094440. PMID 788000.
- Aichinger G, Behner O, Hoffmeister F, Schütz S. Basische tricyclische Oxyiminoäther und ihre pharmakologischen Eigenschaften [Basic tricyclic oxyiminoethers and their pharmacological properties]. Arzneimittelforschung. 1969 Jun;19:Suppl 5a:838+. German. PMID: 5819763.
- Wylie, B. B., Isaacson, E. I., Delgado, J. N. (September 1965). "Synthesis of Oxime Esters and Ethers as Potential Psychotropic Agents". Journal of Pharmaceutical Sciences. 54 (9): 1373–1376. doi:10.1002/jps.2600540932. ISSN 0022-3549.
- Dr-Ing Hermann Engelhard, Dr Norbert Engelhard, & Dipl-Chem Brigitte Werth, DE 1198353 & (1965 to HERMANN ENGELHARD DR ING).
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| Classes | |
|---|---|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.