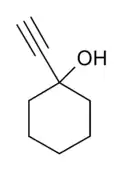
1-Ethynylcyclohexanol
1-Ethynylcyclohexanol (ECX) is an alkynyl alcohol derivative which is both a synthetic precursor to, and an active metabolite of the tranquilizer ethinamate, and has similar sedative, anticonvulsant and muscle relaxant effects. It has been sold as a designer drug, first being identified in the UK in March 2012.[1][2][3][4]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.001 |
| Chemical and physical data | |
| Formula | C8H12O |
| Molar mass | 124.183 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 30–33 °C (86–91 °F) |
SMILES
| |
InChI
| |
Preparation
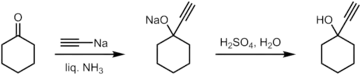
Synthesis of 1-ethynylcyclohexanol from cyclohexanone.
1-Ethynylcyclohexanol can be prepared from cyclohexanone by the reacting it with sodium acetylide in liquid ammonia, followed by an acidic work-up.[5]
See also
- 1,6-Dioxecane-2,7-dione
- 2-Methyl-2-butanol
- 2-Methyl-2-pentanol
- 3-Methyl-3-pentanol
- Clocental
- Ethchlorvynol
- Methylpentynol
- Prenderol
References
- Levina RY, Vinogradova EU (1936). "Action of sodium acetylide on cyclic ketones. I. Synthesis of 1-ethynylcyclohexanol". Zhurnal Prikladnoi Khimii. Sankt-Peterburg, Russian Federation. 9: 1299–1302. ISSN 0044-4618.
- Verkruijsse HD, Graaf WD, Brandsma L (February 1988). "An efficient and quick laboratory scale method for the ethynylation of some aliphatic and cycloaliphatic carbonyl compounds". Synthetic Communications. 18 (2): 131–4. doi:10.1080/00397918808077336.
- Nazarov IN, Kotlyarevskii IL, Ryabchenko VF (1953). "Acetylene derivatives. CLX. Condensation of aldehydes and ketones with acetylene under pressure. New method of synthesis of acetylenic alcohols". Zhurnal Obshchei Khimii. 23: 1900–1904. ISSN 0044-460X.
- "Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA (New drugs in Europe, 2012)" (PDF). Lisbon: EMCDDA. May 2013.
- Saunders JH, Schreiber RS, Jenner EL (1949). "1-ETHYNYLCYCLOHEXANOL". Organic Syntheses. 29: 47. doi:10.15227/orgsyn.029.0047.
Wikimedia Commons has media related to 1-Ethynylcyclohexanol.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.