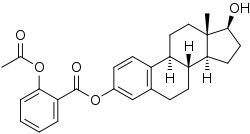
Estradiol acetylsalicylate
 | |
| Clinical data | |
|---|---|
| Other names | Estradiol 3-acetylsalicylate; Acetylsalicylate estradiol |
| Routes of administration | By mouth |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H30O5 |
| Molar mass | 434.532 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Estradiol acetylsalicylate, or estradiol 3-acetylsalicylate, is a synthetic estrogen and estrogen ester – specifically, the C3 acetylsalicylic acid (aspirin) ester of estradiol – which was described in the late 1980s and was never marketed.[1][2][3][4][5] In dogs, the oral bioavailability of estradiol acetylsalicylate was found to be 17-fold higher than that of unmodified estradiol.[1][3] However, a subsequent study found that the oral bioavailability of estradiol and estradiol acetylsalicylate did not differ significantly in rats (4.3% and 4.2%, respectively), suggestive of a major species difference.[2][4][6]
See also
References
- 1 2 Hussain MA, Aungst BJ, Shefter E (January 1988). "Prodrugs for improved oral beta-estradiol bioavailability". Pharm. Res. 5 (1): 44–7. doi:10.1023/A:1015863412137. PMID 3244608. S2CID 7308414.
- 1 2 Lokind, Kenneth B.; Lorenzen, Finn Hjort; Bundgaard, Hans (1991). "Oral bioavailability of 17β-estradiol and various ester prodrugs in the rat". International Journal of Pharmaceutics. 76 (1–2): 177–182. doi:10.1016/0378-5173(91)90356-S. ISSN 0378-5173.
- 1 2 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 263–. ISBN 978-3-642-60107-1.
- 1 2 Valentino Stella; Ronald Borchardt; Michael Hageman; Reza Oliyai, Hans Maag, Jefferson Tilley (26 August 2007). Prodrugs: Challenges and Rewards. Springer Science & Business Media. pp. 347–. ISBN 978-0-387-49785-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Jarkko Rautio (11 January 2011). Prodrugs and Targeted Delivery: Towards Better ADME Properties. John Wiley & Sons. pp. 218–. ISBN 978-3-527-63318-0.
- ↑ Hansen, Joan; Mørk, Niels; Bundgaard, Hans (1992). "Phenyl carbamates of amino acids as prodrug forms for protecting phenols against first-pass metabolism". International Journal of Pharmaceutics. 81 (2–3): 253–261. doi:10.1016/0378-5173(92)90017-V. ISSN 0378-5173.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.