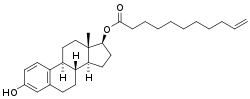
Estradiol undecylenate
 | |
| Clinical data | |
|---|---|
| Other names | SH-368; Estradiol undecenoate; Estra-1,3,5(10)-triene-3,17β-diol 17β-(10-undecenoate) |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.080.203 |
| Chemical and physical data | |
| Formula | C29H42O3 |
| Molar mass | 438.652 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Estradiol undecylenate (EUe; developmental code name SH-368)[1] is an estrogen medication and estrogen ester which was never marketed.[2] It is the C17β undecenoate (undecylenate) ester of estradiol.[2] Following an intramuscular injection, EUe has a very prolonged effect, exceeding that of other estradiol esters like estradiol valerate and estradiol enanthate.[3] Due to its very long duration of action, EUe releases only subthreshold amounts of estradiol at conventional doses.[3] However, this may still be useful in menopausal hormone therapy.[3]
See also
References
- ↑ Unlisted Drugs. Pharmaceutical Section, Special Libraries Association. 1975. ISBN 978-0-913210-02-4.
estradiol undecylate [...] Delestrec [...] SQ 9993 [...] estradiol undecylenate [...] SH 368
- 1 2 DE 1096904, Ringold HJ, Batres E, Rosenkranz G, "Estradiol 17-undecenoate", published 12 January 1961, assigned to Svntex S.A.
- 1 2 3 Harper NJ (1962). "Drug Latentiation". In Jucker E (ed.). Fortschritte der Arzneimittelforschung / Progress in Drug Research / Progrès des recherches pharmaceutiques. Birkhäuser. pp. 243–. ISBN 978-3-0348-7044-3.
Retarded estrogens. In animal experiments it has been shown esterification at C-17 results in longer retarding effects than esterification at C-3. The optimal retarding effect (exceeding 29 days) may be obtained with the C-17 oenanthate. However, the effect exceeds the time interval of 28 days normally considered sufficient for the treatment of a female during one period and for this reason the shorter active estradiol-17-valerianate has been introduced. The estradiol undecylenate has a more protracted effect but it releases only subthreshold doses of steroid (advantage may be taken of this for the treatment of menopause).
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.