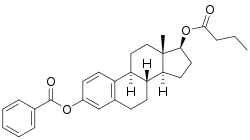
Estradiol benzoate butyrate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Neolutin N, Redimen, Soluna, Unijab (all combinations) |
| Other names | EBB; Estradiol 3-benzoate 17β-n-butyrate; Estra-1,3,5(10)-triene-3,17β-diol 3-benzoate 17β-n-butyrate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.057.989 |
| Chemical and physical data | |
| Formula | C29H34O4 |
| Molar mass | 446.587 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Estradiol benzoate butyrate (EBB), sold under the brand names Neolutin N, Redimen, Soluna, and Unijab and formerly known under the developmental code name Unimens, is an estrogen medication which is used in hormonal birth control for women.[1][2] It is formulated in combination with dihydroxyprogesterone acetophenide (DHPA; algestone acetophenide), a progestin, and is used specifically as a combined injectable contraceptive.[1][2] EBB is not available for medical use alone.[3] The medication, in combination with DHPA, is given by injection into muscle once a month.[1][2]
Side effects of EBB include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[4] EBB is a synthetic estrogen and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[5][6] It is an estrogen ester and a prodrug of estradiol in the body.[6][5] Because of this, it is considered to be a natural and bioidentical form of estrogen.[6]
EBB was first described in 1938.[7] It was developed for use as a form of birth control in the 1970s[8][1] and was introduced for medical use for this indication by the 1980s.[9][10] The medication is used in combination with DHPA as a combined injectable contraceptive in Peru and Singapore.[11][12]
Medical uses
EBB is used in combination with DHPA as a once-a-month combined injectable contraceptive to prevent pregnancy in women.[1][11][12][2][13]
Available forms
The combination of EBB and DHPA contains 10 mg estradiol benzoate butyrate (EBB), an estrogen, and 150 mg algestone acetophenide (dihydroxyprogesterone acetophenide; DHPA), a progestin.[11][12]
Side effects
The combination of EBB and DHPA is said to be associated with poor control of menstrual bleeding when used as a once-a-month combined injectable contraceptive.[1][14]
Pharmacology
Pharmacodynamics
EBB is an estradiol ester, or a prodrug of estradiol.[6][5] As such, it is an estrogen, or an agonist of the estrogen receptors.[6][5] EBB is of about 64% higher molecular weight than estradiol due to the presence of its C3 benzoate and C17β butyrate esters. Because EBB is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[6]
The estrogenic potency of oral ethinylestradiol is approximately 30-fold higher than that of parenteral EBB.[1] In accordance, 50 μg/day oral ethinylestradiol has been reported to be about 3 times stronger in estrogenic effect than once-a-month injections of 10 mg EBB.[1]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Pharmacokinetics
A single 10 mg intramuscular injection of EBB has a duration of approximately 3 weeks.[1][2][15] Its duration is shorter than that of estradiol enantate.[1][2] A preliminary study of the duration of EBB relative to other estradiol esters was conducted in 1952.[16]
Chemistry
EBB is a synthetic estrane steroid and the C3 benzoate (benzenecarboxylate) and C17β butyrate (butanoate) diester of estradiol.[17] It is also known as estradiol 3-benzoate 17β-n-butyrate or as estra-1,3,5(10)-triene-3,17β-diol 3-benzoate 17β-n-butyrate.[17]
The experimental octanol/water partition coefficient (logP) of EBB is 6.3.[18]
| Estrogen | Structure | Ester(s) | Relative mol. weight | Relative E2 contentb | log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzenecarboxylic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
EBB, along with a variety of other estradiol esters, was first described in 1938 by Karl Miescher and colleagues of Ciba in Basel, Switzerland.[7][19][20][21] It was developed in combination with DHPA as a combined injectable contraceptive in the 1970s.[8][1][22][23][24][25] The combination was marketed for use as a combined injectable contraceptive in Peru by 1987.[9][10]
Society and culture
Brand names
EBB is marketed in combination with DHPA under the brand names Neolutin N, Redimen, Soluna, and Unijab.[1][11][12][26][27][28][29] It was originally developed under the tentative brand name Unimens, but ultimately was not marketed under this particular brand name.[1][2][13][8][30]
Availability
The combination of EBB and DHPA is available only in Peru and Singapore.[11][12]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Toppozada M (1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- 1 2 3 4 5 6 7 Mokhtar K. Toppozada (1983). "Monthly Injectable Contraceptives". In Alfredo Goldsmith; Mokhtar Toppozada (eds.). Long-Acting Contraception. pp. 93–103. OCLC 35018604.
- ↑ "Estradiol: Uses, Dosage & Side Effects".
- ↑ Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- 1 2 3 4 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- 1 2 3 4 5 6 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- 1 2 Miescher K, Scholz C, Tschopp E (April 1938). "The activation of female sex hormones: alpha-Oestradiol and its di-esters". Biochem. J. 32 (4): 725–32. doi:10.1042/bj0320725. PMC 1264097. PMID 16746680.
- 1 2 3 Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- 1 2 Bonnema, Jorien; Dalebout, Joanneke A. (1992). "The abuse of high dose estrogen/progestin combination drugs in delay of menstruation: The assumptions and practices of doctors, midwives and pharmacists in a peruvian city". Social Science & Medicine. 34 (3): 281–289. doi:10.1016/0277-9536(92)90270-Z. ISSN 0277-9536. PMID 1557669.
- 1 2 Thomas, David B.; Molina, Ramiro; Cuevas, Hector Rodriguez; Ray, Roberta M.; Riotton, Gustave; Dabancens, Alfredo; Benavides, Socorro; Martinez, Luis; Salas, Oriana; Pallet, Jose A.; Lopez, Jorge (1989). "Monthly injectable steroid contraceptives and cervical carcinoma". American Journal of Epidemiology. 130 (2): 237–247. doi:10.1093/oxfordjournals.aje.a115330. ISSN 1476-6256. PMID 2665476.
- 1 2 3 4 5 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 433, 467. ISBN 978-92-832-1291-1.
- 1 2 3 4 5 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.
- 1 2 Elsayed Saad Eldin Hafez (1980). Human reproduction: conception and contraception. Harper and Row. ISBN 978-0-06-141066-6.
- ↑ Toppozada, Mokhtar K. (1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. ISSN 0010-7824. PMID 8013216.
- ↑ Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 36–. ISBN 978-1-4832-7299-3.
- ↑ Ferin J (January 1952). "Relative duration of action of natural and synthetic estrogens administered parenterally in women with estrogen deficiency". J. Clin. Endocrinol. Metab. 12 (1): 28–35. doi:10.1210/jcem-12-1-28. PMID 14907837.
- 1 2 Edith Josephy; F. Radt (1946). Elsevier's Encyclopaedia of Organic Chemistry: Tetracyclic and higher-cyclic compounds. Elsevier. pp. 99, 680.
- ↑ "Β-Estradiol-3-benzoate 17-N-butyrate | C29H34O4 | ChemSpider".
- ↑ Korenchevsky V, Burbank R, Hall K (March 1939). "The action of the dipropionate and benzoate-butyrate of oestradiol on ovariectomized rats". Biochem. J. 33 (3): 366–71. doi:10.1042/bj0330366. PMC 1264384. PMID 16746921.
- ↑ Sir Norman Lockyer (1938). Nature. Macmillan Journals Limited. p. 292.
The oestradiol benzoate butyrate and dipropionate were supplied by Dr. Miescher (of Ciba Ltd.) who recently described their prolonged effects in rats8.
- ↑ American journal of cancer. 1940.
Note: Our thanks are due to Doctor Karl Miescher of Messrs. Ciba in Basel, Switzerland, for a liberal supply of different esters of estradiol used in this work.
- ↑ Minucci D, Arreghini G, Rabasso A (1973). "Modificazioni endometriali durante trattamento con l'associazione di diidrossiprogesterone acetofenide ed estradiolo-3-benzoato-17-n-butirrato" [Modification of the endometrium during combined therapy with dihydroxyprogesterone acetophenide and estradiol-3-benzoate-17-n-butyrate]. Riv Ostet Ginecol Prat Med Perinat (in Italian). 54 (10): 497–505. PMID 4807299.
- ↑ Cittadini E, Catalano G (1973). "L'impiego di una nuova associazione: diidrossiprogesterone acetofenide ed estradiolo-3-benzoato-17 isobutirrato in ginecologia" [Use of a new combination: dihydroxyprogesterone acetophenide and estradiol-3-benzoate-17 isobutyrate in gynecology]. Riv Ostet Ginecol Prat Med Perinat (in Italian). 54 (10): 506–12. PMID 4620236.
- ↑ Selvaggi L, Putignano G (December 1975). "La risposta dei recettori periferici alla somministrazione parenterale dell'associazione diidrossiprogesterone acetofenide estradiolo-3-benzoato-17n-butirrato. Nota preventiva" [Response of peripheral receptors to the parenteral administration of an association of dihydroxyprogesterone acetophenide and estradiol-3 benzoate-17-n-butyrate. Preliminary note]. Minerva Ginecol (in Italian). 27 (12): 961–3. PMID 778679.
- ↑ Cappello F (December 1975). "L'impiego per via parenterale di un estroprogestinico come inibitore dell'ovulazione in una unica somministrazione mensile" [Use of a parenteral estroprogestin as an inhibitor of ovulation in a single monthly administration]. Minerva Ginecol (in Italian). 27 (12): 964–8. PMID 778680.
- ↑ "Farmaco SOLUNA 150 + 10 registrado en Perú".
- ↑ "Unijab Dosage & Drug Information | MIMS Singapore".
- ↑ http://www.corporacionmisalud.com/sistema/vademecum/PLM/productos/32499.htm
- ↑ https://www.drugs.com/international/soluna.html
- ↑ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.