Feminizing hormone therapy
| Part of a series on |
| Transgender topics |
|---|
|
|
Feminizing hormone therapy, also known as transfeminine hormone therapy, is hormone therapy and sex reassignment therapy to change the secondary sexual characteristics of transgender people from masculine or androgynous to feminine.[1][2][3][4][5][6] It is a common type of transgender hormone therapy (another being masculinizing hormone therapy) and is used to treat transgender women and non-binary transfeminine individuals. Some, in particular, intersex people, but also some cisgender people also take this form of therapy, according to their personal needs and preferences.
The purpose of the therapy is to cause the development of the secondary sex characteristics of the desired sex, such as breasts and a feminine pattern of hair, fat, and muscle distribution. It cannot undo many of the changes produced by naturally occurring puberty, which may necessitate surgery and other treatments to reverse (see below). The medications used for feminizing hormone therapy include estrogens, antiandrogens, progestogens, and gonadotropin-releasing hormone modulators (GnRH modulators).
Feminizing hormone therapy has been shown to likely reduce the distress and discomfort associated with gender dysphoria.[7][8][9]
Requirements
Many physicians operate by the World Professional Association of Transgender Health (WPATH) Standards of Care (SoC) model and require psychotherapy and a letter of recommendation from a psychotherapist in order for a transgender person to obtain hormone therapy.[10] Other physicians operate by an informed consent model and have no requirements for transgender hormone therapy aside from consent.[10] Medications used in transgender hormone therapy are also sold without a prescription on the Internet by unregulated online pharmacies, and some transgender women purchase these medications and treat themselves using a do-it-yourself (DIY) or self-medication approach.[11][12] Many transgender individuals discuss and share information on DIY hormone therapy on Reddit communities such as /r/TransDIY and /r/MtFHRT.[11][12][13][14] One reason that many transgender people turn to DIY hormone therapy is due to long waiting lists of up to years for standard physician-based hormone therapy in some parts of the world such as the United Kingdom, as well as due to the often high costs of seeing a physician and the restrictive criteria that make some ineligible for treatment.[11][12]
The accessibility of transgender hormone therapy differs throughout the world and throughout individual countries.[10]
Medications
| Medication | Brand name | Type | Route | Dosage[lower-alpha 2] |
|---|---|---|---|---|
| Estradiol | Various | Estrogen | Oral | 2–10 mg/day |
| Various | Estrogen | Sublingual | 1–8 mg/day | |
| Climara[lower-alpha 3] | Estrogen | TD patch | 25–400 μg/day | |
| Divigel[lower-alpha 3] | Estrogen | TD gel | 0.5–5 mg/day | |
| Various | Estrogen | SC implant | 50–200 mg every 6–24 mos | |
| Estradiol valerate | Progynova | Estrogen | Oral | 2–10 mg/day |
| Progynova | Estrogen | Sublingual | 1–8 mg/day | |
| Delestrogen[lower-alpha 3] | Estrogen | IM, SC | 2–10 mg/wk or 5–20 mg every 2 wks | |
| Estradiol cypionate | Depo-Estradiol | Estrogen | IM, SC | 2–10 mg/wk or 5–20 mg every 2 wks |
| Estradiol dipropionate | Agofollin | Estrogen | IM, SC | 2–10 mg/wk or 5–20 mg every 2 wks |
| Estradiol benzoate | Progynon-B | Estrogen | IM, SC | 0.5–1.5 mg every 2–3 days |
| Estriol | Ovestin[lower-alpha 3] | Estrogen | Oral | 4–6 mg/day |
| Spironolactone | Aldactone | Antiandrogen | Oral | 100–400 mg/day |
| Cyproterone acetate | Androcur | Antiandrogen; Progestogen | Oral | 5–100 mg/day |
| Androcur Depot | IM | 300 mg/month | ||
| Bicalutamide | Casodex | Antiandrogen | Oral | 25–50 mg/day |
| Enzalutamide | Xtandi | Antiandrogen | Oral | 160 mg/day |
| GnRH analogue | Various | GnRH modulator | Various | Variable |
| Elagolix | Orilissa | GnRH antagonist | Oral | 150 mg/day or 200 mg twice daily |
| Finasteride | Propecia | 5αR inhibitor | Oral | 1–5 mg/day |
| Dutasteride | Avodart | 5αR inhibitor | Oral | 0.25–0.5 mg/day |
| Progesterone | Prometrium[lower-alpha 3] | Progestogen | Oral | 100–400 mg/day |
| Medroxyprogesterone acetate | Provera | Progestogen | Oral | 2.5–40 mg/day |
| Depo-Provera | Progestogen | IM | 150 mg every 3 mos | |
| Depo-SubQ Provera 104 | Progestogen | SC | 104 mg every 3 mos | |
| Hydroxyprogesterone caproate | Proluton | Progestogen | IM | 250 mg/wk |
| Dydrogesterone | Duphaston | Progestogen | Oral | 20 mg/day |
| Drospirenone | Slynd | Progestogen | Oral | 3 mg/day |
| Domperidone[lower-alpha 4] | Motilium | Prolactin releaser | Oral | 30–80 mg/day[lower-alpha 5] |
| ||||
A variety of different sex-hormonal medications are used in feminizing hormone therapy for transgender women.[15][10][3][4] These include estrogens to induce feminization and suppress testosterone levels; antiandrogens such as androgen receptor antagonists, antigonadotropins, GnRH modulators, and 5α-reductase inhibitors to further oppose the effects of androgens like testosterone; and progestogens for various possible though uncertain benefits.[15][10][3][4] An estrogen in combination with an antiandrogen is the mainstay of feminizing hormone therapy for transgender women.[48][49]
Estrogens
Estrogens are the major sex hormones in women, and are responsible for the development and maintenance of feminine secondary sexual characteristics, such as breasts, wide hips, and a feminine pattern of fat distribution.[4] Estrogens act by binding to and activating the estrogen receptor (ER), their biological target in the body.[52] A variety of different forms of estrogens are available and used medically.[52] The most common estrogens used in transgender women include estradiol, which is the predominant natural estrogen in women, and estradiol esters such as estradiol valerate and estradiol cypionate, which are prodrugs of estradiol.[15][4][52] Conjugated estrogens (Premarin), which are used in menopausal hormone therapy, and ethinylestradiol, which is used in birth control pills, have been used in transgender women in the past, but are no longer recommended and are rarely used today due to their higher risks of blood clots and cardiovascular problems.[4][15][10][5] Estrogens may be administered orally, sublingually, transdermally/topically (via patch or gel), rectally, by intramuscular or subcutaneous injection, or by an implant.[52][53][54][55][56] Parenteral (non-oral) routes are preferred, owing to a minimal or negligible risk of blood clots and cardiovascular issues.[5][57][58][59][60]
In addition to producing feminization, estrogens have antigonadotropic effects and suppress gonadal sex hormone production.[53][51][61] They are mainly responsible for the suppression of testosterone levels in transgender women.[53][61] Levels of estradiol of 200 pg/mL and above suppress testosterone levels by about 90%, while estradiol levels of 500 pg/mL and above suppress testosterone levels by about 95%, or to an equivalent extent as surgical castration and GnRH modulators.[62][63] Lower levels of estradiol can also considerably but incompletely suppress testosterone production.[51] When testosterone levels are insufficiently suppressed by estradiol alone, antiandrogens can be used to suppress or block the effects of residual testosterone.[53] Oral estradiol often has difficulty adequately suppressing testosterone levels, due to the relatively low estradiol levels achieved with it.[51][64][65]
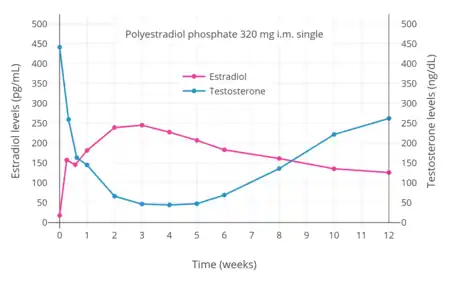
Prior to orchiectomy (surgical removal of the gonads) or sex reassignment surgery, the doses of estrogens used in transgender women are often higher than replacement doses used in cisgender women.[66][67][68] This is to help suppress testosterone levels.[67] The Endocrine Society (2017) recommends maintaining estradiol levels roughly within the normal average range for premenopausal women of about 100 to 200 pg/mL.[15] However, it notes that these physiological levels of estradiol are usually unable to suppress testosterone levels into the female range.[15] A 2018 Cochrane review proposal questioned the notion of keeping estradiol levels lower in transgender women, which results in incomplete suppression of testosterone levels and necessitates the addition of antiandrogens.[69] The review proposal noted that high-dose parenteral estradiol is known to be safe.[69] The Endocrine Society itself recommends dosages of injected estradiol esters that result in estradiol levels markedly in excess of the normal female range, for instance 10 mg per week estradiol valerate by intramuscular injection.[15] A single such injection results in estradiol levels of about 1,250 pg/mL at peak and levels of around 200 pg/mL after 7 days.[70][71] Dosages of estrogens can be reduced after an orchiectomy or sex reassignment surgery, when gonadal testosterone suppression is no longer needed.[5]
Antiandrogens
Antiandrogens are medications that prevent the effects of androgens in the body.[72][73] Androgens, such as testosterone and dihydrotestosterone (DHT), are the major sex hormones in individuals with testes, and are responsible for the development and maintenance of masculine secondary sex characteristics, such as a deep voice, broad shoulders, and a masculine pattern of hair, muscle, and fat distribution.[74][75] In addition, androgens stimulate sex drive and the frequency of spontaneous erections and are responsible for acne, body odor, and androgen-dependent scalp hair loss.[74][75] They also have functional antiestrogenic effects in the breasts and oppose estrogen-mediated breast development, even at low levels.[76][77][78][79] Androgens act by binding to and activating the androgen receptor, their biological target in the body.[80] Antiandrogens work by blocking androgens from binding to the androgen receptor and/or by inhibiting or suppressing the production of androgens.[72]
Antiandrogens that directly block the androgen receptor are known as androgen receptor antagonists or blockers, while antiandrogens that inhibit the enzymatic biosynthesis of androgens are known as androgen synthesis inhibitors and antiandrogens that suppress androgen production in the gonads are known as antigonadotropins.[73] Estrogens and progestogens are antigonadotropins and hence are functional antiandrogens.[53][81][82][83] The purpose of the use of antiandrogens in transgender women is to block or suppress residual testosterone that is not suppressed by estrogens alone.[53][72][61] Additional antiandrogen therapy is not necessarily required if testosterone levels are in the normal female range or if the person has undergone orchiectomy.[53][72][61] However, individuals with testosterone levels in the normal female range and with persisting androgen-dependent skin and/or hair symptoms, such as acne, seborrhea, oily skin, or scalp hair loss, can potentially still benefit from the addition of an antiandrogen, as antiandrogens can reduce or eliminate such symptoms.[84][85][86]
Steroidal antiandrogens
Steroidal antiandrogens are antiandrogens that resemble steroid hormones like testosterone and progesterone in chemical structure.[87] They are the most commonly used antiandrogens in transgender women.[10] Spironolactone (Aldactone), which is relatively safe and inexpensive, is the most frequently used antiandrogen in the United States.[88][89] Cyproterone acetate (Androcur), which is unavailable in the United States, is widely used in Europe, Canada, and the rest of the world.[10][72][88][90] Medroxyprogesterone acetate (Provera, Depo-Provera), a similar medication, is sometimes used in place of cyproterone acetate in the United States.[91][92]
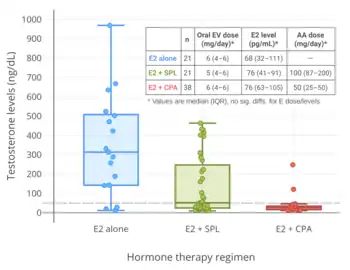
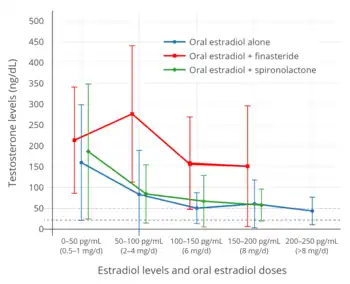
Spironolactone is an antimineralocorticoid (antagonist of the mineralocorticoid receptor) and potassium-sparing diuretic, which is mainly used to treat high blood pressure, edema, high aldosterone levels, and low potassium levels caused by other diuretics, among other uses.[94] Spironolactone is an antiandrogen as a secondary and originally unintended action.[94] It works as an antiandrogen mainly by acting as an androgen receptor antagonist.[95] The medication is also a weak steroidogenesis inhibitor, and inhibits the enzymatic synthesis of androgens.[96][95][97] However, this action is of low potency, and spironolactone has mixed and inconsistent effects on hormone levels.[96][95][97][98][99] In any case, testosterone levels are usually unchanged by spironolactone.[96][95][97][98][99] Studies in transgender women have found testosterone levels to be unaltered with spironolactone[51] or to be decreased.[93] Spironolactone is described as a relatively weak antiandrogen.[100][101][102] It is widely used in the treatment of acne, excessive hair growth, and hyperandrogenism in women, who have much lower testosterone levels than men.[98][99] Because of its antimineralocorticoid activity, spironolactone has antimineralocorticoid side effects[103] and can cause high potassium levels.[104][105] Hospitalization and/or death can potentially result from high potassium levels due to spironolactone,[104][105][106] but the risk of high potassium levels in people taking spironolactone appears to be minimal in those without risk factors for it.[99][107][108] As such, monitoring of potassium levels may not be necessary in most cases.[99][107][108] Spironolactone has been found to decrease the bioavailability of high doses of oral estradiol.[51] Although widely employed, the use of spironolactone as an antiandrogen in transgender women has recently been questioned due to the various shortcomings of the medication for such purposes.[51]
Cyproterone acetate is an antiandrogen and progestin which is used in the treatment of numerous androgen-dependent conditions and is also used as a progestogen in birth control pills.[109][110] It works primarily as an antigonadotropin, secondarily to its potent progestogenic activity, and strongly suppresses gonadal androgen production.[109][61] Cyproterone acetate at a dosage of 5 to 10 mg/day has been found to lower testosterone levels in men by about 50 to 70%,[111][112][113][114] while a dosage of 100 mg/day has been found to lower testosterone levels in men by about 75%.[115][116] The combination of 25 mg/day cyproterone acetate and a moderate dosage of estradiol has been found to suppress testosterone levels in transgender women by about 95%.[117] In combination with estrogen, 10, 25, and 50 mg/day cyproterone acetate have all shown the same degree of testosterone suppression.[118] In addition to its actions as an antigonadotropin, cyproterone acetate is an androgen receptor antagonist.[109][72] However, this action is relatively insignificant at low dosages, and is more important at the high doses of cyproterone acetate that are used in the treatment of prostate cancer (100–300 mg/day).[119][120] Cyproterone acetate can cause elevated liver enzymes and liver damage, including liver failure.[72][121] However, this occurs mostly in prostate cancer patients who take very high doses of cyproterone acetate; liver toxicity has not been reported in transgender women.[72] Cyproterone acetate also has a variety of other adverse effects, such as fatigue and weight gain, and risks, such as blood clots and benign brain tumors, among others.[61][72][122] Periodic monitoring of liver enzymes and prolactin levels may be advisable during cyproterone acetate therapy.
Medroxyprogesterone acetate is a progestin that is related to cyproterone acetate and is sometimes used as an alternative to it.[91][92] It is specifically used as an alternative to cyproterone acetate in the United States, where cyproterone acetate is not approved for medical use and is unavailable.[91][92] Medroxyprogesterone acetate suppresses testosterone levels in transgender women similarly to cyproterone acetate.[92][51] Oral medroxyprogesterone acetate has been found to suppress testosterone levels in men by about 30 to 75% across a dosage range of 20 to 100 mg/day.[123][124][125][126][127] In contrast to cyproterone acetate however, medroxyprogesterone acetate is not also an androgen receptor antagonist.[52][128] Medroxyprogesterone acetate has similar side effects and risks as cyproterone acetate, but is not associated with liver problems.[129][103]
Numerous other progestogens and by extension antigonadotropins have been used to suppress testosterone levels in men and are likely useful for such purposes in transgender women as well.[130][131][132][133][134][135][136] Progestogens alone are in general able to suppress testosterone levels in men by a maximum of about 70 to 80%, or to just above female/castrate levels when used at sufficiently high doses.[137][138][139] The combination of a sufficient dosage of a progestogen with very small doses of an estrogen (e.g., as little as 0.5–1.5 mg/day oral estradiol) is synergistic in terms of antigonadotropic effect and is able to fully suppress gonadal testosterone production, reducing testosterone levels to the female/castrate range.[140][141]
Nonsteroidal antiandrogens
Nonsteroidal antiandrogens are antiandrogens which are nonsteroidal and hence unrelated to steroid hormones in terms of chemical structure.[87][142] These medications are primarily used in the treatment of prostate cancer,[142] but are also used for other purposes such as the treatment of acne, excessive facial/body hair growth, and high androgen levels in women.[19][143][144][145] Unlike steroidal antiandrogens, nonsteroidal antiandrogens are highly selective for the androgen receptor and act as pure androgen receptor antagonists.[142][146] Similarly to spironolactone however, they do not lower androgen levels, and instead work exclusively by preventing androgens from activating the androgen receptor.[142][146] Nonsteroidal antiandrogens are more efficacious androgen receptor antagonists than are steroidal antiandrogens,[87][147] and for this reason, in conjunction with GnRH modulators, have largely replaced steroidal antiandrogens in the treatment of prostate cancer.[142][148]
The nonsteroidal antiandrogens that have been used in transgender women include the first-generation medications flutamide (Eulexin), nilutamide (Anandron, Nilandron), and bicalutamide (Casodex).[19][24][5][3][149]: 477 Newer and even more efficacious second-generation nonsteroidal antiandrogens like enzalutamide (Xtandi), apalutamide (Erleada), and darolutamide (Nubeqa) also exist, but are very expensive due to generics being unavailable and have not been used in transgender women.[150][151] Flutamide and nilutamide have relatively high toxicity, including considerable risks of liver damage and lung disease.[152][143] Due to its risks, the use of flutamide in cisgender and transgender women is now limited and discouraged.[19][143][5] Flutamide and nilutamide have largely been superseded by bicalutamide in clinical practice,[153][154] with bicalutamide accounting for almost 90% of nonsteroidal antiandrogen prescriptions in the United States by the mid-2000s.[155][146] Bicalutamide is said to have excellent tolerability and safety relative to flutamide and nilutamide, as well as in comparison to cyproterone acetate.[156][157][158] It has few to no side effects in women.[144][145] Despite its greatly improved tolerability and safety profile however, bicalutamide does still have a small risk of elevated liver enzymes and association with very rare cases of liver damage and lung disease.[19][152][159]
Nonsteroidal antiandrogens like bicalutamide may be a particularly favorable option for transgender women who wish to preserve sex drive, sexual function, and/or fertility, relative to antiandrogens that suppress testosterone levels and can greatly disrupt these functions such as cyproterone acetate and GnRH modulators.[160][161][162] However, estrogens suppress testosterone levels and at high doses can markedly disrupt sex drive and function and fertility on their own.[163][164][165][166] Moreover, disruption of gonadal function and fertility by estrogens may be permanent after extended exposure.[165][166]
GnRH modulators
GnRH modulators are antigonadotropins and hence functional antiandrogens.[167] In both males and females, gonadotropin-releasing hormone (GnRH) is produced in the hypothalamus and induces the secretion of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland.[167] The gonadotropins signal the gonads to make sex hormones such as testosterone and estradiol.[167] GnRH modulators bind to and inhibit the GnRH receptor, thereby preventing gonadotropin release.[167] As a result of this, GnRH modulators are able to completely shut-down gonadal sex hormone production, and can decrease testosterone levels in men and transgender women by about 95%, or to an equivalent extent as surgical castration.[167][168][169] GnRH modulators are also commonly known as GnRH analogues.[167] However, not all clinically used GnRH modulators are analogues of GnRH.[170]
There are two types of GnRH modulators: GnRH agonists and GnRH antagonists.[167] These medications have the opposite action on the GnRH receptor but paradoxically have the same therapeutic effects.[167] GnRH agonists, such as leuprorelin (Lupron), goserelin (Zoladex), and buserelin (Suprefact), are GnRH receptor superagonists, and work by producing profound desensitization of the GnRH receptor such that the receptor becomes non-functional.[167][168] This occurs because GnRH is normally released in pulses, but GnRH agonists are continuously present, and this results in excessive downregulation of the receptor and ultimately a complete loss of function.[171][172][167] At the initiation of treatment, GnRH agonists are associated with a "flare" effect on hormone levels due to acute overstimulation of the GnRH receptor.[167][173] In men, LH levels increase by up to 800%, while testosterone levels increase to about 140 to 200% of baseline.[174][173] Gradually however, the GnRH receptor desensitizes; testosterone levels peak after about 2 to 4 days, return to baseline after about 7 to 8 days, and are reduced to castrate levels within 2 to 4 weeks.[173] Antigonadotropins such as estrogens and cyproterone acetate as well as nonsteroidal antiandrogens such as flutamide and bicalutamide can be used beforehand and concomitantly to reduce or prevent the effects of the testosterone flare caused by GnRH agonists.[175][174][176][177][53][178] In contrast to GnRH agonists, GnRH antagonists, such as degarelix (Firmagon) and elagolix (Orilissa), work by binding to the GnRH receptor without activating it, thereby displacing GnRH from the receptor and preventing its activation.[167] Unlike with GnRH agonists, there is no initial surge effect with GnRH antagonists; the therapeutic effects are immediate, with sex hormone levels being reduced to castrate levels within a few days.[167][168]
GnRH modulators are highly effective for testosterone suppression in transgender women and have few or no side effects when sex hormone deficiency is avoided with concomitant estrogen therapy.[15][179] However, GnRH modulators tend to be very expensive (typically US$10,000 to US$15,000 per year in the United States), and are often denied by medical insurance.[15][180][181][182] GnRH modulator therapy is much less economical than surgical castration, and is less convenient than surgical castration in the long-term as well.[183] Because of their costs, many transgender women cannot afford GnRH modulators and must use other, often less effective options for testosterone suppression.[15][180] GnRH agonists are prescribed as standard practice for transgender women in the United Kingdom however, where the National Health Service (NHS) covers them.[180][184] This is in contrast to the rest of Europe and to the United States.[184] Another drawback of GnRH modulators is that most of them are peptides and are not orally active, requiring administration by injection, implant, or nasal spray.[176] However, non-peptide and orally active GnRH antagonists, elagolix (Orilissa) and relugolix (Relumina), were introduced for medical use in 2018 and 2019, respectively. But they are under patent protection and, as with other GnRH modulators, are very expensive at present.[185]
In adolescents of either sex with relevant indicators, GnRH modulators can be used to stop undesired pubertal changes for a period without inducing any changes toward the sex with which the patient currently identifies. There is considerable controversy over the earliest age at which it is clinically, morally, and legally safe to use GnRH modulators, and for how long. The sixth edition of the World Professional Association for Transgender Health's Standards of Care permit it from Tanner stage 2 but do not allow the addition of hormones until age 16, which could be five or more years later. Sex steroids have important functions in addition to their role in puberty, and some skeletal changes (such as increased height) that may be considered masculine are not hindered by GnRH modulators.
5α-Reductase inhibitors
5α-Reductase inhibitors are inhibitors of the enzyme 5α-reductase, and are a type of specific androgen synthesis inhibitor.[186][187] 5α-Reductase is an enzyme that is responsible for the conversion of testosterone into the more potent androgen dihydrotestosterone (DHT).[186][187] There are three different isoforms of 5α-reductase, types 1, 2, and 3, and these three isoforms show different patterns of expression in the body.[186] Relative to testosterone, DHT is about 2.5- to 10-fold more potent as an agonist of the androgen receptor.[186][187][188] As such, 5α-reductase serves to considerably potentiate the effects of testosterone.[186][187] However, 5α-reductase is expressed only in specific tissues, such as skin, hair follicles, and the prostate gland, and for this reason, conversion of testosterone into DHT happens only in certain parts of the body.[186][187][189] Furthermore, circulating levels of total and free DHT in men are very low at about 1/10th and 1/20th those of testosterone, respectively,[187][190][186] and DHT is efficiently inactivated into weak androgens in various tissues such as muscle, fat, and liver.[186][168][191] As such, it is thought that DHT plays little role as a systemic androgen hormone and serves more as a means of locally potentiating the androgenic effects of testosterone in a tissue-specific manner.[186][192][193] Conversion of testosterone into DHT by 5α-reductase plays an important role in male reproductive system development and maintenance (specifically of the penis, scrotum, prostate gland, and seminal vesicles), male-pattern facial/body hair growth, and scalp hair loss, but has little role in other aspects of masculinization.[186][187][189][194][195] Besides the involvement of 5α-reductase in androgen signaling, it is also required for the conversion of steroid hormones such as progesterone and testosterone into neurosteroids like allopregnanolone and 3α-androstanediol, respectively.[196][197]
5α-Reductase inhibitors include finasteride and dutasteride.[186][187] Finasteride is a selective inhibitor of 5α-reductase types 2 and 3, while dutasteride is an inhibitor of all three isoforms of 5α-reductase.[186][198][199] Finasteride can decrease circulating DHT levels by up to 70%, whereas dutasteride can decrease circulating DHT levels by up to 99%.[198][199] Conversely, 5α-reductase inhibitors do not decrease testosterone levels, and may actually increase them slightly.[15][51][61][200] 5α-Reductase inhibitors are used primarily in the treatment of benign prostatic hyperplasia, a condition in which the prostate gland becomes excessively large due to stimulation by DHT and causes unpleasant urogenital symptoms.[198][201] They are also used in the treatment of androgen-dependent scalp hair loss in men and women.[202][203][204] The medications are able to prevent further scalp hair loss in men and can restore some scalp hair density.[202][203][205] Conversely, the effectiveness of 5α-reductase inhibitors in the treatment of scalp hair loss in women is less clear.[204][187] This may be because androgen levels are much lower in women, in whom they may not play as important of a role in scalp hair loss.[204][187] 5α-Reductase inhibitors are also used to treat hirsutism (excessive body/facial hair growth) in women, and are very effective for this indication.[206] Dutasteride has been found to be significantly more effective than finasteride in the treatment of scalp hair loss in men, which has been attributed to its more complete inhibition of 5α-reductase and by extension decrease in DHT production.[207][208][142] In addition to their antiandrogenic uses, 5α-reductase inhibitors have been found to reduce adverse affective symptoms in premenstrual dysphoric disorder in women.[209][210] This is thought to be due to prevention by 5α-reductase inhibitors of the conversion of progesterone into allopregnanolone during the luteal phase of the menstrual cycle.[209][210]
5α-Reductase inhibitors are sometimes used as a component of feminizing hormone therapy for transgender women in combination with estrogens and/or other antiandrogens.[4][211][68] They may have beneficial effects limited to improvement of scalp hair loss, body hair growth, and possibly skin symptoms such as acne.[212][10][213][68] However, little clinical research on 5α-reductase inhibitors in transgender women has been conducted, and evidence of their efficacy and safety in this group is limited.[211][33] Moreover, 5α-reductase inhibitors have only mild and specific antiandrogenic activity, and are not recommended as general antiandrogens.[33]
5α-Reductase inhibitors have minimal side effects and are well tolerated in both men and women.[214][215] In men, the most common side effect is sexual dysfunction (0.9–15.8% incidence), which may include decreased libido, erectile dysfunction, and reduced ejaculate.[214][215][216][217][218] Another side effect in men is breast changes, such as breast tenderness and gynecomastia (2.8% incidence).[215] Due to decreased levels of androgens and/or neurosteroids, 5α-reductase inhibitors may slightly increase the risk of depression (~2.0% incidence).[217][219][220][214][197] There are reports that a small percentage of men may experience persistent sexual dysfunction and adverse mood changes even after discontinuation of 5α-reductase inhibitors.[218][221][219][222][217][216][197] Some of the possible side effects of 5α-reductase inhibitors in men, such as gynecomastia and sexual dysfunction, are actually welcome changes for many transgender women.[19] In any case, caution may be warranted in using 5α-reductase inhibitors in transgender women, as this group is already at a high risk for depression and suicidality.[223][61]
Progestogens
Progesterone, a progestogen, is the other of the two major sex hormones in women.[176] It is mainly involved in the regulation of the female reproductive system, the menstrual cycle, pregnancy, and lactation.[176] The non-reproductive effects of progesterone are fairly insignificant.[224] Unlike estrogens, progesterone is not known to be involved in the development of female secondary sexual characteristics, and hence is not believed to contribute to feminization in women.[10][92] One area of particular interest in terms of the effects of progesterone in women is breast development.[225][226][227] Estrogens are responsible for the development of the ductal and connective tissues of the breasts and the deposition of fat into the breasts during puberty in girls.[225][226] Conversely, high levels of progesterone, in conjunction with other hormones such as prolactin, are responsible for the lobuloalveolar maturation of the mammary glands during pregnancy.[225][226] This allows for lactation and breastfeeding after childbirth.[225][226] Although progesterone causes the breasts to change during pregnancy, the breasts undergo involution and revert to their pre-pregnancy composition and size after the cessation of breastfeeding.[225][228][226] Every pregnancy, lobuloalveolar maturation occurs again anew.[225][226]
There are two types of progestogens: progesterone, which is the natural and bioidentical hormone in the body; and progestins, which are synthetic progestogens.[52] There are dozens of clinically used progestins.[52][229][230] Certain progestins, namely cyproterone acetate and medroxyprogesterone acetate and as described previously, are used at high doses as functional antiandrogens due to their antigonadotropic effects to help suppress testosterone levels in transgender women.[91][92] Aside from the specific use of testosterone suppression however, there are no other indications of progestogens in transgender women at present.[10] In relation to this, the use of progestogens in transgender women is controversial, and they are not otherwise routinely prescribed or recommended.[10][5][6][231][33][232] Besides progesterone, cyproterone acetate, and medroxyprogesterone acetate, other progestogens that have been reported to have been used in transgender women include hydroxyprogesterone caproate, dydrogesterone, norethisterone acetate, and drospirenone.[233][234][33][235][5][236] Progestins in general largely have the same progestogenic effects however, and in theory, any progestin could be used in transgender women.[52]
Clinical research on the use of progestogens in transgender women is very limited.[10][227] Some patients and clinicians believe, on the basis of anecdotal and subjective claims, that progestogens may provide benefits such as improved breast and/or nipple development, mood, and libido in transgender women.[4][3][227] There are no clinical studies to support such reports at present.[10][4][227] No clinical study has assessed the use of progesterone in transgender women, and only a couple of studies have compared the use of progestins (specifically cyproterone acetate and medroxyprogesterone acetate) versus the use of no progestogen in transgender women.[227][237][179] These studies, albeit limited in the quality of their findings, reported no benefit of progestogens on breast development in transgender women.[227][179][231] This has also been the case in limited clinical experience.[238] These reports are in accordance with the normal and even above-average breast development in women with complete androgen insensitivity syndrome, who lack progesterone and have no lobuloalveolar development of the mammary glands on histological examination.[76][239] It is noteworthy that epithelial tissue, which makes up lobuloalveolar tissue, normally (outside of pregnancy and lactation) comprises only about 10 to 15% of the tissue of the breasts.[240][241][242][243] Although the influence of progesterone on breast development is uncertain, progesterone is thought to cause reversible breast enlargement during the menstrual cycle due to local fluid retention in the breasts.[244][245] This may give a misleading appearance of breast growth, and might contribute to anecdotal reports of improved breast size and/or shape with progesterone in transgender women.[244][245]
Progestogens have some antiestrogenic effects in the breasts, for instance decreasing expression of the estrogen receptor and increasing expression of estrogen-metabolizing enzymes,[246][247][248][249] and for this reason, have been used to treat breast pain and benign breast disorders.[250][251][252][253] Progesterone levels during female puberty do not normally increase importantly until near the end of puberty in cisgender girls, a point by which most breast development has already been completed.[254] In addition, concern has been expressed that premature exposure to progestogens during the process of breast development is unphysiological and might compromise final breast growth outcome, although this notion presently remains theoretical.[19][227][255] Though the role of progestogens in pubertal breast development is uncertain, progesterone is essential for lobuloalveolar maturation of the mammary glands during pregnancy.[225] Hence, progestogens are required for any transgender woman who wishes to lactate or breastfeed.[45][256][227] A study found full lobuloalveolar maturation of the mammary glands on histological examination in transgender women treated with an estrogen and high-dose cyproterone acetate.[257][258][259] However, lobuloalveolar development reversed with discontinuation of cyproterone acetate, indicating that continued progestogen exposure is necessary to maintain the tissue.[257]
In terms of the effects of progestogens on sex drive, one study assessed the use of dydrogesterone to improve sexual desire in transgender women and found no benefit.[235] Another study likewise found that oral progesterone did not improve sexual function in cisgender women.[260]
Progestogens can have adverse effects.[231][33][52][229][261][55] Oral progesterone has inhibitory neurosteroid effects and can produce side effects such as sedation, mood changes, and alcohol-like effects.[52][262][263] Many progestins have off-target activity, such as androgenic, antiandrogenic, glucocorticoid, and antimineralocorticoid activity, and these activities likewise can contribute unwanted side effects.[52][229] Furthermore, the addition of a progestin to estrogen therapy has been found to increase the risk of blood clots, cardiovascular disease (e.g., coronary heart disease and stroke), and breast cancer compared to estrogen therapy alone in postmenopausal women.[264][33][231][265] Although it is unknown if these health risks of progestins occur in transgender women similarly, it cannot be ruled out that they do.[264][33][231] High-dose progestogens increase the risk of benign brain tumors including prolactinomas and meningiomas as well.[266][267] Because of their potential detrimental effects and lack of supported benefits, some researchers have argued that, aside from the purpose of testosterone suppression, progestogens should not generally be used or advocated in transgender women or should only be used for a limited duration (e.g., 2–3 years).[264][231][5][6][232] Conversely, other researchers have argued that the risks of progestogens in transgender women are likely minimal, and that in light of potential albeit hypothetical benefits, should be used if desired.[3] In general, some transgender women respond favorably to the effects of progestogens, while others respond negatively.[3]
Progesterone is most commonly taken orally.[52][265] However, oral progesterone has very low bioavailability, and produces relatively weak progestogenic effects even at high doses.[268][269][265][270][271] In accordance, and in contrast to progestins, oral progesterone has no antigonadotropic effects in men even at high doses.[262][272] Progesterone can also be taken by various parenteral (non-oral) routes, including sublingually, rectally, and by intramuscular or subcutaneous injection.[52][252][273] These routes do not have the bioavailability and efficacy issues of oral progesterone, and accordingly, can produce considerable antigonadotropic and other progestogenic effects.[52][270][274] Transdermal progesterone is poorly effective, owing to absorption issues.[52][252][271] Progestins are usually taken orally.[52] In contrast to progesterone, most progestins have high oral bioavailability, and can produce full progestogenic effects with oral administration.[52] Some progestins, such as medroxyprogesterone acetate and hydroxyprogesterone caproate, are or can be used by intramuscular or subcutaneous injection instead.[275][252] Almost all progestins, with the exception of dydrogesterone, have antigonadotropic effects.[52]
Miscellaneous
Galactogogues such as the peripherally selective D2 receptor antagonist and prolactin releaser domperidone can be used to induce lactation in transgender women who wish to breastfeed.[276][277][45] An extended period of combined estrogen and progestogen therapy is necessary to mature the lobuloalveolar tissue of the breasts before this can be successful.[256][45][278][257] There are several published reports of lactation and/or breastfeeding in transgender women.[279][280][256][278][45][281][282]
Interactions
Many of the medications used in feminizing hormone therapy, such as estradiol, cyproterone acetate, and bicalutamide, are substrates of CYP3A4 and other cytochrome P450 enzymes. As a result, inducers of CYP3A4 and other cytochrome P450 enzymes, such as carbamazepine, phenobarbital, phenytoin, rifampin, rifampicin, and St. John's wort, among others, may decrease circulating levels of these medications and thereby decrease their effects. Conversely, inhibitors of CYP3A4 and other cytochrome P450 enzymes, such as cimetidine, clotrimazole, grapefruit juice, itraconazole, ketoconazole, and ritonavir, among others, may increase circulating levels of these medications and thereby increase their effects. The concomitant use of a cytochrome P450 inducer or inhibitor with feminizing hormone therapy may necessitate medication dosage adjustments.
Effects
The spectrum of effects of hormone therapy in transfeminine people depend on the specific medications and dosages used. In any case, the main effects of hormone therapy in transfeminine people are feminization and demasculinization, and are as follows:
| Effect | Time to expected onset of effect[lower-alpha 1] | Time to expected maximum effect[lower-alpha 1][lower-alpha 2] | Permanency if hormone therapy is stopped |
|---|---|---|---|
| Breast development and nipple/areolar enlargement | 2–6 months | 1–5 years | Permanent |
| Thinning/slowed growth of facial/body hair | 4–12 months | >3 years[lower-alpha 3] | Reversible |
| Cessation/reversal of male-pattern scalp hair loss | 1–3 months | 1–2 years[lower-alpha 4] | Reversible |
| Softening of skin/decreased oiliness and acne | 3–6 months | Unknown | Reversible |
| Redistribution of body fat in a feminine pattern | 3–6 months | 2–5 years | Reversible |
| Decreased muscle mass/strength | 3–6 months | 1–2 years[lower-alpha 5] | Reversible |
| Widening and rounding of the pelvis[lower-alpha 6] | Unspecified | Unspecified | Permanent |
| Changes in mood, emotionality, and behavior | Unspecified | Unspecified | Reversible |
| Decreased sex drive | 1–3 months | 3–6 months | Reversible |
| Decreased spontaneous/morning erections | 1–3 months | 3–6 months | Reversible |
| Erectile dysfunction and decreased ejaculate volume | 1–3 months | Variable | Reversible |
| Decreased sperm production/fertility | Unknown | >3 years | Reversible or permanent[lower-alpha 7] |
| Decreased testicle size | 3–6 months | 2–3 years | Unknown |
| Decreased penis size | None[lower-alpha 8] | Not applicable | Not applicable |
| Decreased prostate gland size | Unspecified | Unspecified | Unspecified |
| Voice changes | None[lower-alpha 9] | Not applicable | Not applicable |
Footnotes and sources
Footnotes:
| |||
Physical changes
Breast development
Breast, nipple, and areolar development varies considerably depending on genetics, body composition, age of HRT initiation, and many other factors. Development can take a couple years to nearly a decade for some. However, many transgender women report there is often a "stall" in breast growth during transition, or significant breast asymmetry. Transgender women on HRT often experience less breast development than cisgender women (especially if started after young adulthood). For this reason, many seek breast augmentation. Transgender patients opting for breast reduction are rare. Shoulder width and the size of the rib cage also play a role in the perceivable size of the breasts; both are usually larger in transgender women, causing the breasts to appear proportionally smaller. Thus, when a transgender woman opts to have breast augmentation, the implants used tend to be larger than those used by cisgender women.[291]
In clinical trials, cisgender women have used stem cells from fat to regrow their breasts after mastectomies. This could some day eliminate the need for implants for transgender women.[292]
In transgender women on HRT, as in cisgender women during puberty, breast ducts and Cooper's ligaments develop under the influence of estrogen. Progesterone causes the milk sacs (mammary alveoli) to develop, and with the right stimuli, a transgender woman may lactate. Additionally, HRT often makes the nipples more sensitive to stimulation.
Breast development in transgender women begins within two to three months of the start of hormone therapy and continues for up to two years.[293][213] Breast development seems to be better in transgender women who have a higher body mass index.[293][213] As a result, it may be beneficial to breast development for thin transgender women to gain some weight in the early phases of hormone therapy.[293][213] Different estrogens, such as estradiol valerate, conjugated estrogens, and ethinylestradiol, appear to produce equivalent results in terms of breast sizes in transgender women.[293][237][179] The sudden discontinuation of estrogen therapy has been associated with onset of galactorrhea (lactation).[293][213]
Skin changes
The uppermost layer of skin, the stratum corneum, becomes thinner and more translucent. Spider veins may appear or be more noticeable as a result. Collagen decreases, and tactile sensation increases. The skin becomes softer,[294] more susceptible to tearing and irritation from scratching or shaving, and slightly lighter in color because of a slight decrease in melanin.
Sebaceous gland activity (which is triggered by androgens) lessens, reducing oil production on the skin and scalp. Consequently, the skin becomes less prone to acne. It also becomes drier, and lotions or oils may be necessary.[291][295] The pores become smaller because of the lower quantities of oil being produced. Many apocrine glands – a type of sweat gland – become inactive, and body odor decreases. Remaining body odor becomes less metallic, sharp, or acrid, and more sweet and musky.
As subcutaneous fat accumulates,[291] dimpling, or cellulite, becomes more apparent on the thighs and buttocks. Stretch marks (striae distensae) may appear on the skin in these areas. Susceptibility to sunburn increases, possibly because the skin is thinner and less pigmented.
Hair changes
Antiandrogens affect existing facial hair only slightly; patients may see slower growth and some reduction in density and coverage. Those who are less than a decade past puberty and/or lack a significant amount of facial hair may have better results. Patients taking antiandrogens tend to have better results with electrolysis and laser hair removal than those who are not. In patients in their teens or early twenties, antiandrogens prevent new facial hair from developing if testosterone levels are within the normal female range.[291][295]
Body hair (on the chest, shoulders, back, abdomen, buttocks, thighs, tops of hands, and tops of feet) turns, over time, from terminal ("normal") hairs to tiny, blonde vellus hairs. Arm, perianal, and perineal hair is reduced but may not turn to vellus hair on the latter two regions (some cisgender women also have hair in these areas). Underarm hair changes slightly in texture and length, and pubic hair becomes more typically female in pattern. Lower leg hair becomes less dense. All of these changes depend to some degree on genetics.[291][295]
Head hair may change slightly in texture, curl, and color. This is especially likely with hair growth from previously bald areas. Eyebrows do not change because they are not androgenic hair.[296]
Eye changes
The lens of the eye changes in curvature.[297][298][299][294] Because of decreased androgen levels, the meibomian glands (the sebaceous glands on the upper and lower eyelids that open up at the edges) produce less oil. Because oil prevents the tear film from evaporating, this change may cause dry eyes.[300][301][302][303][304]
Fat changes
The distribution of adipose (fat) tissue changes slowly over months and years. HRT causes the body to accumulate new fat in a typically feminine pattern, including in the hips, thighs, buttocks, pubis, upper arms, and breasts. The body begins to burn old adipose tissue in the waist, shoulders, and back, making those areas smaller.[291]
Subcutaneous fat increases in the cheeks and lips, making the face appear rounder, with slightly less emphasis on the jaw as the lower portion of the cheeks fills in.
Bone/skeletal changes
If estrogen therapy is begun at an early age, widening of the hips may occur.
Unaffected characteristics
HRT does not reverse bone changes that have already been established by puberty. Consequently, it does not affect height except for the aforementioned reasons; the length of the arms, legs, hands, and feet; or the width of the shoulders and rib cage. However, details of bone shape change throughout life, with bones becoming heavier and more deeply sculptured under the influence of androgens, and HRT does prevent such changes from progressing further.
The width of the hips is not affected in individuals for whom epiphyseal closure (fusion and closure of the ends of bones, which prevents any further lengthening) has taken place. This occurs in most people between 18 and 25 years of age. Already-established changes to the shape of the hips cannot be reversed by HRT whether epiphyseal closure has taken place or not.
Established changes to the bone structure of the face are also unaffected by HRT. A significant majority of craniofacial changes occur during adolescence. Post-adolescent growth is considerably slower and minimal by comparison.[305] Also unaffected is the prominence of the thyroid cartilage (Adam's apple). These changes may be reversed by surgery (facial feminization surgery and tracheal shave, respectively).
During puberty, the voice deepens in pitch and becomes more resonant. These changes are permanent and are not affected by HRT. Voice therapy and/or surgery may be used instead to achieve a more female-sounding voice.
Facial hair develops during puberty and is only slightly affected by HRT. It may, however, be eliminated nearly permanently with laser hair removal, or permanently with electrolysis.
Mental changes
The psychological effects of feminizing hormone therapy are harder to define than physical changes. Because hormone therapy is usually the first physical step taken to transition, the act of beginning it has a significant psychological effect, which is difficult to distinguish from hormonally induced changes.
Mood changes
Changes in mood and well-being occur with hormone therapy in transgender women.[306]
Sexual changes
Some transgender women report a significant reduction in libido, depending on the dosage of antiandrogens.[307] A small number of post-operative transgender women take low doses of testosterone to boost their libido. Raising the dosage of estrogen or adding a progestogen raises the libido of some transgender women.
Spontaneous and morning erections decrease significantly in frequency, although some patients who have had an orchiectomy still experience morning erections. Voluntary erections may or may not be possible, depending on the amount of hormones and/or antiandrogens being taken.
Managing long-term hormonal regimens have not been studied and are difficult to estimate because research on the long-term use of hormonal therapy has not been noted.[264] However, it is possible to speculate the outcomes of these therapies on transgender people based on the knowledge of the current effects of gonadal hormones on sexual functioning in cisgender men and women.[308]
Firstly, if one is to decrease testosterone in feminizing gender transition, it is likely that sexual desire and arousal would be inhibited; alternatively, if high doses of estrogen negatively impact sexual desire, which has been found in some research with cisgender women, it is hypothesized that combining androgens with high levels of estrogen would intensify this outcome.[308] Unfortunately, to date there haven't been any randomized clinical trials looking at the relationship between type and dose of transgender hormone therapy, so the relationship between them remains unclear.[308] Typically, the estrogens given for feminizing gender transition are 2 to 3 times higher than the recommended dose for HRT in postmenopausal women.[264] Pharmacokinetic studies indicate taking these increased doses may lead to a higher boost in plasma estradiol levels; however, the long-term side effects haven't been studied and the safety of this route is unclear.[264]
As with any pharmacological or hormone therapy, there are potential side effects, which in the case of transgender hormone therapy include changes in sexual functioning. These have the ability to significantly impact sexual functioning, either directly or indirectly through the various side effects, such as cerebrovascular disorders, obesity, and mood fluctuations.[308] In addition, some research has found an onset of diabetes following feminizing hormone therapy, which impairs sexual response. Whatever route an individual and their doctor choose to take, it is important to consider both the medical risks of hormone therapy as well as the psychological needs of the patient.
Brain changes
Several studies have found that hormone therapy in transgender women causes the structure of the brain to change in the direction of female proportions.[309][310][311][312][313] In addition, studies have found that hormone therapy in transgender women causes performance in cognitive tasks, including visuospatial, verbal memory, and verbal fluency, to shift in a more female direction.[309][306]
Adverse effects
Cardiovascular effects
The most significant cardiovascular risk for transgender women is the prothrombotic effect (increased blood clotting) of estrogens. This manifests most significantly as an increased risk for venous thromboembolism (VTE): deep vein thrombosis (DVT) and pulmonary embolism (PE), which occurs when blood clots from DVT break off and migrate to the lungs. Symptoms of DVT include pain or swelling of one leg, especially the calf. Symptoms of PE include chest pain, shortness of breath, fainting, and heart palpitations, sometimes without leg pain or swelling.
VTE occurs more frequently in the first year of treatment with estrogens. The risk of VTE is higher with oral non-bioidentical estrogens such as ethinylestradiol and conjugated estrogens than with parenteral formulations of estradiol such as injectable, transdermal, implantable, and intranasal.[314][315][316][317][318][319][320][321][322][323][324][166][325][326][327][328][329][59][330][331][332][333] VTE risk also increases with age and in patients who smoke, so many clinicians advise using the safer estrogen formulations in smokers and patients older than 40. In addition, VTE risk is increased by progestins and increases with the dosages of both estrogens and progestins. Obesity increases the risk of VTE as well. Increased risk of VTE with estrogens is thought to be due to their influence on liver protein synthesis, specifically on the production of coagulation factors.[52] Non-bioidentical estrogens such as conjugated estrogens and especially ethinylestradiol have markedly disproportionate effects on liver protein synthesis relative to estradiol.[52] In addition, oral estradiol has a 4- to 5-fold increased impact on liver protein synthesis than does transdermal estradiol and other parenteral estradiol routes.[52][334]
Because the risks of warfarin – which is used to treat blood clots – in a relatively young and otherwise healthy population are low, while the risk of adverse physical and psychological outcomes for untreated transgender patients is high, prothrombotic mutations (such as factor V Leiden, antithrombin III, and protein C or S deficiency) are not absolute contraindications for hormonal therapy.[213]
A 2018 cohort study of 2842 transfeminine individuals in the United States treated with a mean follow-up of 4.0 years observed an increased risk of VTE, stroke, and heart attack relative to a cisgender reference population.[335][336][19][58] The estrogens used included oral estradiol (1 to 10 mg/day) and other estrogen formulations.[58] Other medications such as antiandrogens like spironolactone were also used.[58]
A 2019 systematic review and meta-analysis found an incidence rate of VTE of 2.3 per 1000 person-years with feminizing hormone therapy in transgender women.[337] For comparison, the rate in the general population has been found to be 1.0–1.8 per 1000 person-years, and the rate in premenopausal women taking birth control pills has been found to be 3.5 per 1000 patient-years.[337][338] There was significant heterogeneity in the rates of VTE across the included studied, and the meta-analysis was unable to perform subgroup analyses between estrogen type, estrogen route, estrogen dosage, concomitant antiandrogen or progestogen use, or patient characteristics (e.g., sex, age, smoking status, weight) corresponding to known risk factors for VTE.[337] Due to the inclusion of some studies using ethinylestradiol, which is more thrombotic and is no longer used in transgender women, the researchers noted that the VTE risk found in their study may be an overestimate.[337]
In a 2016 study that specifically assessed oral estradiol, the incidence of VTE in 676 transgender women who were treated for an average of 1.9 years each was only one individual, or 0.15% of the group, with an incidence of 7.8 events per 10,000 person-years.[339][340] The dosage of oral estradiol used was 2 to 8 mg/day.[340] Almost all of the transgender women were also taking spironolactone (94%), a subset were also taking finasteride (17%), and fewer than 5% were also taking a progestogen (usually oral progesterone).[340] The findings of this study suggest that the incidence of VTE is low in transgender women taking oral estradiol.[339][340]
Cardiovascular health in transgender women has been reviewed in recent publications.[341][57]
Gastrointestinal effects
Estrogens may increase the risk of gallbladder disease, especially in older and obese people.[294] They may also increase transaminase levels, indicating liver toxicity, especially when taken in oral form.
Metabolic changes
A patient's metabolic rate may change, causing an increase or decrease in weight and energy levels, changes to sleep patterns, and temperature sensitivity. Androgen deprivation leads to slower metabolism and a loss of muscle tone. Building muscle takes more work. The addition of a progestogen may increase energy, although it may increase appetite as well.
Bone changes
Both estrogens and androgens are necessary in all humans for bone health. Young, healthy women produce about 10 mg of testosterone monthly, and higher bone mineral density in males is associated with higher serum estrogen. Both estrogen and testosterone help to stimulate bone formation, especially during puberty. Estrogen is the predominant sex hormone that slows bone loss, regardless of sex.
Cancer risk
Studies are mixed on whether the risk of breast cancer is increased with hormone therapy in transgender women.[342][343][344][345] Two cohort studies found no increase in risk relative to cisgender men,[343][344] whereas another cohort study found an almost 50-fold increase in risk such that the incidence of breast cancer was between that of cisgender men and cisgender women.[345][342] There is no evidence that breast cancer risk in transgender women is greater than in cisgender women.[346] Twenty cases of breast cancer in transgender women have been reported as of 2019.[342][347]
Cisgender men with gynecomastia have not been found to have an increased risk of breast cancer.[348] It has been suggested that a 46,XY karyotype (one X chromosome and one Y chromosome) may be protective against breast cancer compared to having a 46,XX karyotype (two X chromosomes).[348] Men with Klinefelter's syndrome (47,XXY karyotype), which causes hypoandrogenism, hyperestrogenism, and a very high incidence of gynecomastia (80%), have a dramatically (20- to 58-fold) increased risk of breast cancer compared to karyotypical men (46,XY), closer to the rate of karyotypical women (46,XX).[348][349][350] The incidences of breast cancer in karyotypical men, men with Klinefelter's syndrome, and karyotypical women are approximately 0.1%,[351] 3%,[349] and 12.5%,[352] respectively. Women with complete androgen insensitivity syndrome (46,XY karyotype) never develop male sex characteristics and have normal and complete female morphology, including breast development,[353] yet have not been reported to develop breast cancer.[74][354] The risk of breast cancer in women with Turner syndrome (45,XO karyotype) also appears to be significantly decreased, though this could be related to ovarian failure and hypogonadism rather than to genetics.[355]
Prostate cancer is extremely rare in gonadectomized transgender women who have been treated with estrogens for a prolonged period of time.[15][356][357] Whereas as many as 70% of men show prostate cancer by their 80s,[154] only a handful of cases of prostate cancer in transgender women have been reported in the literature.[15][356][357] As such, and in accordance with the fact that androgens are responsible for the development of prostate cancer, HRT appears to be highly protective against prostate cancer in transgender women.[15][356][357]
The risks of certain types of benign brain tumors including meningioma and prolactinoma are increased with hormone therapy in transgender women.[358] These risks have mostly been associated with the use of cyproterone acetate.[358]
Estrogens and progestogens can cause prolactinomas, which are benign, prolactin-secreting tumors of the pituitary gland. Milk discharge from the nipples can be a sign of elevated prolactin levels. If a prolactinoma becomes large enough, it can cause visual changes (especially decreased peripheral vision), headaches, depression or other mood changes, dizziness, nausea, vomiting, and symptoms of pituitary failure, like hypothyroidism.
Monitoring
Especially in the early stages of feminizing hormone therapy, blood work is done frequently to assess hormone levels and liver function. The Endocrine Society recommends that patients have blood tests every three months in the first year of HRT for estradiol and testosterone, and that spironolactone, if used, be monitored every two to three months in the first year.[15] Recommended ranges for total estradiol and total testosterone levels include but are not limited to the following:
| Source | Place | Estradiol, total | Testosterone, total | |
|---|---|---|---|---|
| Endocrine Society | United States | 100–200 pg/mL | <50 ng/dL | |
| World Professional Association for Transgender Health (WPATH) | United States | "[T]estosterone levels [...] below the upper limit of the normal female range and estradiol levels within a premenopausal female range but well below supraphysiologic levels." "[M]aintain levels within physiologic ranges for a patient's desired gender expression (based on goals of full feminization/masculinization)." | ||
| Center of Excellence for Transgender Health (UCSF) | United States | "The interpretation of hormone levels for transgender individuals is not yet evidence based; physiologic hormone levels in non-transgender people are used as reference ranges." "Providers are encouraged to consult with their local lab(s) to obtain hormone level reference ranges for both 'male' and 'female' norms, [which can vary,] and then apply the correct range when interpreting results based on the current hormonal sex, rather than the sex of registration." | ||
| Fenway Health | United States | 100–200 pg/mL | <55 ng/dL | |
| Callen-Lorde | United States | "Some guidelines recommend checking estradiol and testosterone levels at baseline and throughout the monitoring of estrogen therapy. We have not found a clinical use for routine hormone levels that justifies the expense. However, we recognize that individual providers may adjust their prescribing and monitoring practices as needed to comply with guidelines or when guided by patient need." | ||
| International Planned Parenthood Federation (IPPF) | United Kingdom | <200 pg/mL | 30–100 ng/dL | |
| National Health Service (NHS) Foundation Trusts | United Kingdom | 55–160 pg/mL | 30–85 ng/dL | |
| Royal College of Psychiatry (RCP) | United Kingdom | 80–140 pg/mL | "Well below normal male range" | |
| Vancouver Coastal Health (VCH) | Canada | ND | <45 ng/dL | |
| Sources: See template. | ||||
The optimal ranges for estrogen apply only to individuals taking estradiol (or an ester of estradiol), and not to those taking synthetic or other non-bioidentical preparations (e.g., conjugated estrogens or ethinylestradiol).[15]
Physicians also recommend broader medical monitoring, including complete blood counts; tests of renal function, liver function, and lipid and glucose metabolism; and monitoring of prolactin levels, body weight, and blood pressure.[15][359]
If prolactin levels are greater than 100 ng/mL, estrogen therapy should be stopped and prolactin levels should be rechecked after 6 to 8 weeks.[359] If prolactin levels remain high, an MRI scan of the pituitary gland to check for the presence of a prolactinoma should be ordered.[359] Otherwise, estrogen therapy may be restarted at a lower dosage.[359] Cyproterone acetate is particularly associated with elevated prolactin levels, and discontinuation of cyproterone acetate lowers prolactin levels.[360][361][362] In contrast to cyproterone acetate, estrogen and spironolactone therapy is not associated with increased prolactin levels.[362][363]
History
Effective pharmaceutical female sex-hormonal medications, including androgens, estrogens, and progestogens, first became available in the 1920s and 1930s.[364] One of the earliest reports of hormone therapy in transgender women was published by Danish endocrinologist Christian Hamburger in 1953.[365] One of his patients was Christine Jorgensen, who he had treated starting in 1950.[366][367][368][369] Additional reports of hormone therapy in transgender women were published by Hamburger, the German-American endocrinologist Harry Benjamin, and other researchers in the mid-to-late 1960s.[370][371][372][373][374][375] However, Benjamin had several hundred transgender patients under his care by the late 1950s,[92] and had treated transgender women with hormone therapy as early as the late 1940s or early 1950s.[376][377][378][366] In any case, Hamburger is said to be the first to treat transgender women with hormone therapy.[379]
One of the first transgender health clinics was opened in the mid-1960s at the Johns Hopkins School of Medicine.[380][92] By 1981, there were almost 40 such centers.[381] A review of the hormonal regimens of 20 of the centers was published that year.[370][381] The first International Symposium on Gender Identity, chaired by Christopher John Dewhurst, was held in London in 1969,[382] and the first medical textbook on transgenderism, titled Transsexualism and Sex Reassignment and edited by Richard Green and John Money, was published by Johns Hopkins University Press in 1969.[383][384] This textbook included a chapter on hormone therapy written by Christian Hamburger and Harry Benjamin.[375] The Harry Benjamin International Gender Dysphoria Association (HBIGDA), now known as the World Professional Association for Transgender Health (WPATH), was formed in 1979, with the first version of the Standards of Care published the same year.[366] The Endocrine Society published guidelines for the hormonal care of transgender people in 2009, with a revised version in 2017.[370][385][15]
Hormone therapy for transgender women was initially done using high-dose estrogen therapy with oral estrogens such as conjugated estrogens, ethinylestradiol, and diethylstilbestrol and with parenteral estrogens such as estradiol benzoate, estradiol valerate, estradiol cypionate, and estradiol undecylate.[373][374][375][381][386] Progestogens, such as hydroxyprogesterone caproate, medroxyprogesterone acetate, and other progestins, were also sometimes included.[365][373][374][381][387][39][388] The antiandrogen and progestogen cyproterone acetate was first used in transgender women by 1977.[389][390][391] Its use was standard at the Center of Expertise on Gender Dysphoria (CEGD; Kennis- en Zorgcentrum Genderdysforie, or KZcG) in Amsterdam, the Netherlands by 1985.[392][386] Spironolactone, another antiandrogen, was first used in transgender women by 1986.[393][387][386][287][394] These agents were described as allowing the use of much lower doses of estrogen than previously required and this was considered advantageous due to risks of high doses of estrogens such as cardiovascular complications.[387][386][391] Antiandrogens were well-established in hormone therapy for transgender women by the early 1990s.[39][264][395] Estrogen doses in transgender women were reduced following the introduction of antiandrogens. Ethinylestradiol, conjugated estrogens, and other non-bioidentical estrogens stopped being used in transgender women in favor of estradiol starting around 2000 due to their greater risks of blood clots and cardiovascular issues.[288][341][337]
See also
References
- ↑ Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG (November 2017). "Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline" (PDF). J. Clin. Endocrinol. Metab. 102 (11): 3869–3903. doi:10.1210/jc.2017-01658. PMID 28945902. S2CID 3726467.
- ↑ Coleman, E.; Bockting, W.; Botzer, M.; Cohen-Kettenis, P.; DeCuypere, G.; Feldman, J.; Fraser, L.; Green, J.; Knudson, G.; Meyer, W. J.; Monstrey, S.; Adler, R. K.; Brown, G. R.; Devor, A. H.; Ehrbar, R.; Ettner, R.; Eyler, E.; Garofalo, R.; Karasic, D. H.; Lev, A. I.; Mayer, G.; Meyer-Bahlburg, H.; Hall, B. P.; Pfaefflin, F.; Rachlin, K.; Robinson, B.; Schechter, L. S.; Tangpricha, V.; van Trotsenburg, M.; Vitale, A.; Winter, S.; Whittle, S.; Wylie, K. R.; Zucker, K. (2012). "Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7" (PDF). International Journal of Transgenderism. 13 (4): 165–232. doi:10.1080/15532739.2011.700873. ISSN 1553-2739. S2CID 39664779.
- 1 2 3 4 5 6 7 8 Deutsch M (17 June 2016). "Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People" (PDF) (2nd ed.). University of California, San Francisco: Center of Excellence for Transgender Health. p. 28.
- 1 2 3 4 5 6 7 8 9 10 Wesp LM, Deutsch MB (March 2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". Psychiatr. Clin. North Am. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
- 1 2 3 4 5 6 7 8 9 10 Dahl, M; Feldman, JL; Goldberg, J; Jaberi, A (2015). "Endocrine Therapy for Transgender Adults in British Columbia: Suggested Guidelines" (PDF). Vancouver Coastal Health. Retrieved 15 August 2018.
- 1 2 3 4 5 Bourns, Amy (2015). "Guidelines and Protocols for Comprehensive Primary Care for Trans Clients" (PDF). Sherbourne Health Centre. Retrieved 15 August 2018.
- ↑ Murad, Mohammad Hassan; Elamin, Mohamed B.; Garcia, Magaly Zumaeta; Mullan, Rebecca J.; Murad, Ayman; Erwin, Patricia J.; Montori, Victor M. (2010). "Hormonal therapy and sex reassignment: A systematic review and meta-analysis of quality of life and psychosocial outcomes". Clinical Endocrinology. 72 (2): 214–231. doi:10.1111/j.1365-2265.2009.03625.x. PMID 19473181. S2CID 19590739.
- ↑ White Hughto, Jaclyn M.; Reisner, Sari L. (2016). "A Systematic Review of the Effects of Hormone Therapy on Psychological Functioning and Quality of Life in Transgender Individuals". Transgender Health. 1 (1): 21–31. doi:10.1089/trgh.2015.0008. PMC 5010234. PMID 27595141.
- ↑ Foster Skewis, Lucas; Bretherton, Ingrid; Leemaqz, Shalem Y.; Zajac, Jeffrey D.; Cheung, Ada S. (2021). "Short-Term Effects of Gender-Affirming Hormone Therapy on Dysphoria and Quality of Life in Transgender Individuals: A Prospective Controlled Study". Frontiers in Endocrinology. 12: 717766. doi:10.3389/fendo.2021.717766. PMC 8358932. PMID 34394009.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Coleman, E.; Bockting, W.; Botzer, M.; Cohen-Kettenis, P.; DeCuypere, G.; Feldman, J.; Fraser, L.; Green, J.; Knudson, G.; Meyer, W. J.; Monstrey, S.; Adler, R. K.; Brown, G. R.; Devor, A. H.; Ehrbar, R.; Ettner, R.; Eyler, E.; Garofalo, R.; Karasic, D. H.; Lev, A. I.; Mayer, G.; Meyer-Bahlburg, H.; Hall, B. P.; Pfaefflin, F.; Rachlin, K.; Robinson, B.; Schechter, L. S.; Tangpricha, V.; van Trotsenburg, M.; Vitale, A.; Winter, S.; Whittle, S.; Wylie, K. R.; Zucker, K. (2012). "Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7" (PDF). International Journal of Transgenderism. 13 (4): 165–232. doi:10.1080/15532739.2011.700873. ISSN 1553-2739. S2CID 39664779.
- 1 2 3 Branstetter, Gillian (31 August 2016). "Sketchy Pharmacies Are Selling Hormones to Transgender People: Burdened by cost and medical discrimination, many people are taking a do-it-yourself approach to transitioning". The Atlantic. Retrieved 29 December 2018.
- 1 2 3 Newman, Rosalind; Jeory, Ted (16 November 2016). "Fears of 'DIY transitioning' as hormone drugs sold to transgender women without checks". The Independent. Retrieved 29 December 2018.
- ↑ "r/TransDIY". Reddit. Retrieved 29 December 2018.
- ↑ "r/MtFHRT". Reddit. Retrieved 29 December 2018.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG (November 2017). "Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline" (PDF). J. Clin. Endocrinol. Metab. 102 (11): 3869–3903. doi:10.1210/jc.2017-01658. PMID 28945902. S2CID 3726467.
- ↑ Wylie, Kevan; Barrett, James; Besser, Mike; Bouman, Walter Pierre; Bridgman, Michelle; Clayton, Angela; Green, Richard; Hamilton, Mark; Hines, Melissa; Ivbijaro, Gabriel; Khoosal, Deenesh; Lawrence, Alex; Lenihan, Penny; Loewenthal, Del; Ralph, David; Reed, Terry; Stevens, John; Terry, Tim; Thom, Ben; Thornton, Jane; Walsh, Dominic; Ward, David; Coleman, Eli; Di Ceglie, Domenico; Martin, Emma; McGarry, Philip; Messenger, Andrew; Reid, Russell; Sethi, Su; Sutcliffe, Paul; Wilson, Daniel; Carr, Susan; Davies, Dai; Dean, Tracey; Ellis, Michelle; Ferguson, Brian; Skinner, Darren; Williams, Vicky; Brechin, Susan; Lucey, Jim; Rathbone, Maxine (2014). "Good Practice Guidelines for the Assessment and Treatment of Adults with Gender Dysphoria" (PDF). Sexual and Relationship Therapy. 29 (2): 154–214. doi:10.1080/14681994.2014.883353. ISSN 1468-1994. S2CID 144632597.
- ↑ Wesp LM, Deutsch MB (March 2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". Psychiatr. Clin. North Am. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
- ↑ Unger CA (December 2016). "Hormone therapy for transgender patients". Transl Androl Urol. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
- 1 2 3 4 5 6 7 8 Randolph JF (December 2018). "Gender-Affirming Hormone Therapy for Transgender Females". Clin Obstet Gynecol. 61 (4): 705–721. doi:10.1097/GRF.0000000000000396. PMID 30256230.
- ↑ Nakatsuka M (May 2010). "Endocrine treatment of transsexuals: assessment of cardiovascular risk factors". Expert Rev Endocrinol Metab. 5 (3): 319–322. doi:10.1586/eem.10.18. PMID 30861686. S2CID 73253356.
- ↑ Fishman, Sarah L.; Paliou, Maria; Poretsky, Leonid; Hembree, Wylie C. (2019). "Endocrine Care of Transgender Adults". Transgender Medicine. Contemporary Endocrinology. pp. 143–163. doi:10.1007/978-3-030-05683-4_8. ISBN 978-3-030-05682-7. ISSN 2523-3785.
- ↑ Winkler-Crepaz, K.; Müller, A.; Böttcher, B.; Wildt, L. (2017). "Hormonbehandlung bei Transgenderpatienten" [Hormone treatment of transgender patients]. Gynäkologische Endokrinologie. 15 (1): 39–42. doi:10.1007/s10304-016-0116-9. ISSN 1610-2894. S2CID 12270365.
- ↑ Urdl, W. (2009). "Behandlungsgrundsätze bei Transsexualität" [Therapeutic principles in transsexualism]. Gynäkologische Endokrinologie. 7 (3): 153–160. doi:10.1007/s10304-009-0314-9. ISSN 1610-2894. S2CID 8001811.
- 1 2 Gooren LJ (March 2011). "Clinical practice. Care of transsexual persons". N. Engl. J. Med. 364 (13): 1251–7. doi:10.1056/NEJMcp1008161. PMID 21449788.
- ↑ James Barrett (29 September 2017). Transsexual and Other Disorders of Gender Identity: A Practical Guide to Management. CRC Press. pp. 216–. ISBN 978-1-315-34513-0.
- ↑ Carlo Trombetta; Giovanni Liguori; Michele Bertolotto (3 March 2015). Management of Gender Dysphoria: A Multidisciplinary Approach. Springer. pp. 85–. ISBN 978-88-470-5696-1.
- ↑ Fabris B, Bernardi S, Trombetta C (March 2015). "Cross-sex hormone therapy for gender dysphoria". J. Endocrinol. Invest. 38 (3): 269–82. doi:10.1007/s40618-014-0186-2. PMID 25403429. S2CID 207503049.
- ↑ Kristen Eckstrand; Jesse M. Ehrenfeld (17 February 2016). Lesbian, Gay, Bisexual, and Transgender Healthcare: A Clinical Guide to Preventive, Primary, and Specialist Care. Springer. pp. 357–. ISBN 978-3-319-19752-4.
- ↑ Tangpricha V, den Heijer M (April 2017). "Oestrogen and anti-androgen therapy for transgender women". Lancet Diabetes Endocrinol. 5 (4): 291–300. doi:10.1016/S2213-8587(16)30319-9. PMC 5366074. PMID 27916515.
- ↑ Coxon, Jonny; Seal, Leighton (2018). "Hormone management of trans women". Trends in Urology & Men's Health. 9 (6): 10–14. doi:10.1002/tre.663. ISSN 2044-3730. S2CID 222189278.
- ↑ Gooren LJ, Giltay EJ, Bunck MC (January 2008). "Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience". J. Clin. Endocrinol. Metab. 93 (1): 19–25. doi:10.1210/jc.2007-1809. PMID 17986639.
- ↑ Athanasoulia-Kaspar, Anastasia P.; Stalla, Günter K. (2019). "Endokrinologische Betreuung von Patienten mit Transsexualität" [Endocrinological care of patients with transsexuality]. Geburtshilfe und Frauenheilkunde. 79 (7): 672–675. doi:10.1055/a-0801-3319. ISSN 0016-5751.
- 1 2 3 4 5 6 7 8 Meriggiola MC, Gava G (November 2015). "Endocrine care of transpeople part II. A review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen". Clin. Endocrinol. (Oxf). 83 (5): 607–15. doi:10.1111/cen.12754. PMID 25692882. S2CID 39706760.
- ↑ Costa EM, Mendonca BB (March 2014). "Clinical management of transsexual subjects". Arq Bras Endocrinol Metabol. 58 (2): 188–96. doi:10.1590/0004-2730000003091. PMID 24830596.
- ↑ Moore E, Wisniewski A, Dobs A (August 2003). "Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects". The Journal of Clinical Endocrinology and Metabolism. 88 (8): 3467–73. doi:10.1210/jc.2002-021967. PMID 12915619.
- ↑ Rosenthal SM (December 2014). "Approach to the patient: transgender youth: endocrine considerations". J. Clin. Endocrinol. Metab. 99 (12): 4379–89. doi:10.1210/jc.2014-1919. PMID 25140398.
- ↑ Arver DS (2015). "Transsexualism, könsdysfori". Retrieved 2018-11-12.
- ↑ Bourgeois AL, Auriche P, Palmaro A, Montastruc JL, Bagheri H (February 2016). "Risk of hormonotherapy in transgender people: Literature review and data from the French Database of Pharmacovigilance". Ann. Endocrinol. (Paris). 77 (1): 14–21. doi:10.1016/j.ando.2015.12.001. PMID 26830952.
- 1 2 3 4 Asscheman, Henk; Gooren, Louis J.G. (1993). "Hormone Treatment in Transsexuals". Journal of Psychology & Human Sexuality. 5 (4): 39–54. doi:10.1300/J056v05n04_03. ISSN 0890-7064.
- ↑ Levy A, Crown A, Reid R (October 2003). "Endocrine intervention for transsexuals". Clin. Endocrinol. (Oxf). 59 (4): 409–18. doi:10.1046/j.1365-2265.2003.01821.x. PMID 14510900. S2CID 24493388.
- ↑ Vincenzo Mirone (12 February 2015). Clinical Uro-Andrology. Springer. pp. 17–. ISBN 978-3-662-45018-5.
- ↑ Lim HH, Jang YH, Choi GY, Lee JJ, Lee ES (January 2019). "Gender affirmative care of transgender people: a single center's experience in Korea". Obstet Gynecol Sci. 62 (1): 46–55. doi:10.5468/ogs.2019.62.1.46. PMC 6333764. PMID 30671393.
When we prescribed estradiol, we preferred sublingual estradiol valerate instead of the oral form for feminizing HT since prior researchers have reported the effectiveness of sublingual administration in maintaining high blood estradiol concentration and low E1/E2 ratio [13].
- ↑ Gianna E. Israel (March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 56–. ISBN 978-1-56639-852-7.
- ↑ Majumder, Anirban; Chatterjee, Sudip; Maji, Debasis; Roychaudhuri, Soumyabrata; Ghosh, Sujoy; Selvan, Chitra; George, Belinda; Kalra, Pramila; Maisnam, Indira; Sanyal, Debmalya (2020). "IDEA group consensus statement on medical management of adult gender incongruent individuals seeking gender reaffirmation as female". Indian Journal of Endocrinology and Metabolism. 24 (2): 128. doi:10.4103/ijem.IJEM_593_19. ISSN 2230-8210. PMID 32699777. S2CID 218596936.
- 1 2 3 4 5 Reisman T, Goldstein Z (2018). "Case Report: Induced Lactation in a Transgender Woman". Transgend Health. 3 (1): 24–26. doi:10.1089/trgh.2017.0044. PMC 5779241. PMID 29372185.
- ↑ Henderson A (2003). "Domperidone. Discovering new choices for lactating mothers". Awhonn Lifelines. 7 (1): 54–60. doi:10.1177/1091592303251726. PMID 12674062.
- ↑ "Orilissa (elagolix) FDA Label" (PDF). 24 July 2018. Retrieved 31 July 2018.
- ↑ William B. Shore (21 August 2014). Adolescent Medicine, An Issue of Primary Care: Clinics in Office Practice, E-Book. Elsevier Health Sciences. pp. 663–. ISBN 978-0-323-32340-6.
- ↑ Ivy M. Alexander; Versie Johnson-Mallard; Elizabeth Kostas-Polston; Catherine Ingram Fogel, Nancy Fugate Woods (28 June 2017). Women's Health Care in Advanced Practice Nursing, Second Edition. Springer Publishing Company. pp. 468–. ISBN 978-0-8261-9004-8.
- ↑ Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057.
- 1 2 3 4 5 6 7 8 9 10 Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgend Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- 1 2 3 4 5 6 7 8 Unger CA (December 2016). "Hormone therapy for transgender patients". Transl Androl Urol. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
- ↑ Alfred S. Wolf; H.P.G. Schneider (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 79, 81. ISBN 978-3-642-75101-1.
- 1 2 Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ↑ Lauritzen C (December 1986). "Die Behandlung der klimakterischen Beschwerden durch vaginale, rektale und transdermale Ostrogensubstitution" [Treatment of disorders of the climacteric by vaginal, rectal and transdermal estrogen substitution]. Gynakologe (in German). 19 (4): 248–53. ISSN 0017-5994. PMID 3817597.
- 1 2 Irwig MS (September 2018). "Cardiovascular health in transgender people". Rev Endocr Metab Disord. 19 (3): 243–251. doi:10.1007/s11154-018-9454-3. PMID 30073551. S2CID 51908458.
- 1 2 3 4 Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M (August 2018). "Cross-sex Hormones and Acute Cardiovascular Events in Transgender Persons: A Cohort Study". Ann. Intern. Med. 169 (4): 205–213. doi:10.7326/M17-2785. PMC 6636681. PMID 29987313.
- 1 2 Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer—a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433. S2CID 6847203.
- ↑ Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- 1 2 3 4 5 6 7 8 9 Tangpricha V, den Heijer M (April 2017). "Oestrogen and anti-androgen therapy for transgender women". Lancet Diabetes Endocrinol. 5 (4): 291–300. doi:10.1016/S2213-8587(16)30319-9. PMC 5366074. PMID 27916515.
- ↑ Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384. S2CID 32650111.
- ↑ Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD (May 2003). "Transdermal estradiol therapy for advanced prostate cancer--forward to the past?". J. Urol. 169 (5): 1735–7. doi:10.1097/01.ju.0000061024.75334.40. PMID 12686820.
- ↑ Leinung, MC (June 2014). "Variable Response to Oral Estradiol Therapy in Male to Female Transgender Patients". Endocrine Reviews. 35 (Supplement). doi:10.1210/endo-meetings.2014.RE.2.OR42-1 (inactive 31 October 2021).
{{cite journal}}: CS1 maint: DOI inactive as of October 2021 (link) - ↑ Liang JJ, Jolly D, Chan KJ, Safer JD (February 2018). "Testosterone Levels Achieved by Medically Treated Transgender Women in a United States Endocrinology Clinic Cohort". Endocr Pract. 24 (2): 135–142. doi:10.4158/EP-2017-0116. PMID 29144822.
- ↑ Gooren LJ, Giltay EJ, Bunck MC (January 2008). "Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience". J. Clin. Endocrinol. Metab. 93 (1): 19–25. doi:10.1210/jc.2007-1809. PMID 17986639.
- 1 2 Wylie, Kevan Richard; Fung, Robert; Boshier, Claudia; Rotchell, Margaret (2009). "Recommendations of endocrine treatment for patients with gender dysphoria". Sexual and Relationship Therapy. 24 (2): 175–187. doi:10.1080/14681990903023306. ISSN 1468-1994. S2CID 20471537.
- 1 2 3 Carlo Trombetta; Giovanni Liguori; Michele Bertolotto (3 March 2015). Management of Gender Dysphoria: A Multidisciplinary Approach. Springer. pp. 85–. ISBN 978-88-470-5696-1.
- 1 2 Haupt, Claudia; Henke, Miriam; Kutschmar, Alexia; Hauser, Birgit; Baldinger, Sandra; Schreiber, Gerhard (2018). "Antiandrogens or estradiol treatments or both during hormone replacement therapy in transitioning transgender women". Cochrane Database of Systematic Reviews. 2018 (10): CD013138. doi:10.1002/14651858.CD013138. ISSN 1465-1858. PMC 6517060.
- ↑ Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- ↑ Rauramo L, Punnonen R, Kaihola LH, Grönroos M (January 1980). "Serum oestrone, oestradiol and oestriol concentrations in castrated women during intramuscular oestradiol valerate and oestradiolbenzoate-oestradiolphenylpropionate therapy". Maturitas. 2 (1): 53–8. doi:10.1016/0378-5122(80)90060-2. PMID 7402086.
- 1 2 3 4 5 6 7 8 9 Gava, Giulia; Seracchioli, Renato; Meriggiola, Maria Cristina (2017). "Therapy with Antiandrogens in Gender Dysphoric Natal Males". Endocrinology of the Testis and Male Reproduction. Endocrinology. pp. 1199–1209. doi:10.1007/978-3-319-44441-3_42. ISBN 978-3-319-44440-6. ISSN 2510-1927.
- 1 2 Lieberman R (2001). "Androgen deprivation therapy for prostate cancer chemoprevention: current status and future directions for agent development". Urology. 58 (2 Suppl 1): 83–90. doi:10.1016/s0090-4295(01)01247-x. PMID 11502457.
There are several classes of antiandrogens including (1) antigonadotropins (eg, LHRH agonists/antagonists, synthetic estrogens [diethylstilbestrol]); (2) nonsteroidal androgen-receptor antagonists (eg, flutamide, bicalutamide, nilutamide); (3) steroidal agents with mixed actions (eg, cyproterone acetate); (4) adrenal androgen inhibitors (eg, ketoconazole, hydrocortisone); (5) steroidal agents that inhibit androgen biosynthesis (eg, 5α-reductase inhibitors (type II) and dual-acting 5α-reductase inhibitors); [...]
- 1 2 3 Shlomo Melmed; Kenneth S. Polonsky; P. Reed Larsen; Henry M. Kronenberg (11 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 714, 934. ISBN 978-0-323-34157-8.
- 1 2 Sarah Boslaugh (3 August 2018). Transgender Health Issues. ABC-CLIO. pp. 37–. ISBN 978-1-4408-5888-8.
- 1 2 Jerome F. Strauss; Robert L. Barbieri; Antonio R. Gargiulo (23 December 2017). Yen & Jaffe's Reproductive Endocrinology E-Book: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 250–. ISBN 978-0-323-58232-2.
- ↑ Dimitrakakis C (September 2011). "Androgens and breast cancer in men and women" (PDF). Endocrinol. Metab. Clin. North Am. 40 (3): 533–47, viii. doi:10.1016/j.ecl.2011.05.007. PMID 21889719.
- ↑ Schneider HP (November 2003). "Androgens and antiandrogens". Ann. N. Y. Acad. Sci. 997 (1): 292–306. Bibcode:2003NYASA.997..292S. doi:10.1196/annals.1290.033. PMID 14644837. S2CID 8400556.
- ↑ Tiefenbacher K, Daxenbichler G (2008). "The Role of Androgens in Normal and Malignant Breast Tissue". Breast Care (Basel). 3 (5): 325–331. doi:10.1159/000158055. PMC 2931104. PMID 20824027.
- ↑ Gibson DA, Saunders PK, McEwan IJ (April 2018). "Androgens and androgen receptor: Above and beyond". Mol. Cell. Endocrinol. 465: 1–3. doi:10.1016/j.mce.2018.02.013. PMID 29481861. S2CID 3702165.
- ↑ Brueggemeier, Robert W. (2006). "Sex Hormones (Male): Analogs and Antagonists". Encyclopedia of Molecular Cell Biology and Molecular Medicine. doi:10.1002/3527600906.mcb.200500066. ISBN 978-3527600908.
- ↑ de Lignières B, Silberstein S (April 2000). "Pharmacodynamics of oestrogens and progestogens". Cephalalgia. 20 (3): 200–7. doi:10.1046/j.1468-2982.2000.00042.x. PMID 10997774. S2CID 40392817.
- ↑ Neumann F (1978). "The physiological action of progesterone and the pharmacological effects of progestogens--a short review". Postgraduate Medical Journal. 54 Suppl 2: 11–24. PMID 368741.
- ↑ Lotti, Francesco; Maggi, Mario (2015). "Hormonal Treatment for Skin Androgen-Related Disorders". European Handbook of Dermatological Treatments. pp. 1451–1464. doi:10.1007/978-3-662-45139-7_142. ISBN 978-3-662-45138-0.
- ↑ Schmidt TH, Shinkai K (October 2015). "Evidence-based approach to cutaneous hyperandrogenism in women". J. Am. Acad. Dermatol. 73 (4): 672–90. doi:10.1016/j.jaad.2015.05.026. PMID 26138647.
- ↑ Clapauch, Ruth; Weiss, Rita Vasconcellos; Rech, Ciciliana Maila Zilio (2017). "Testosterone and Women". Testosterone. pp. 319–351. doi:10.1007/978-3-319-46086-4_17. ISBN 978-3-319-46084-0.
- 1 2 3 Singh SM, Gauthier S, Labrie F (2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Curr. Med. Chem. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- 1 2 Loren S Schechter (22 September 2016). Surgical Management of the Transgender Patient. Elsevier Health Sciences. pp. 26–. ISBN 978-0-323-48408-4.
- ↑ Lynne Carroll; Lauren Mizock (7 February 2017). Clinical Issues and Affirmative Treatment with Transgender Clients, An Issue of Psychiatric Clinics of North America, E-Book. Elsevier Health Sciences. pp. 107–. ISBN 978-0-323-51004-2.
- ↑ Laura Erickson-Schroth (12 May 2014). Trans Bodies, Trans Selves: A Resource for the Transgender Community. Oxford University Press. pp. 258–. ISBN 978-0-19-932536-8.
- 1 2 3 4 J. Larry Jameson; Leslie J. De Groot (18 May 2010). Endocrinology - E-Book: Adult and Pediatric. Elsevier Health Sciences. pp. 2282–. ISBN 978-1-4557-1126-0.
- 1 2 3 4 5 6 7 8 Randi Ettner; Stan Monstrey; Eli Coleman (20 May 2016). Principles of Transgender Medicine and Surgery. Routledge. pp. 169–170, 216, 251. ISBN 978-1-317-51460-2.
- 1 2 3 Angus L, Leemaqz S, Ooi O, Cundill P, Silberstein N, Locke P, Zajac JD, Cheung AS (July 2019). "Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy". Endocr Connect. 8 (7): 935–940. doi:10.1530/EC-19-0272. PMC 6612061. PMID 31234145.
- 1 2 Kolkhof P, Bärfacker L (July 2017). "30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development". J. Endocrinol. 234 (1): T125–T140. doi:10.1530/JOE-16-0600. PMC 5488394. PMID 28634268.
- 1 2 3 4 McMullen GR, Van Herle AJ (December 1993). "Hirsutism and the effectiveness of spironolactone in its management". J. Endocrinol. Invest. 16 (11): 925–32. doi:10.1007/BF03348960. PMID 8144871. S2CID 42231952.
- 1 2 3 Loriaux, D. Lynn (November 1976). "Spironolactone and endocrine dysfunction". Annals of Internal Medicine. 85 (5): 630–6. doi:10.7326/0003-4819-85-5-630. PMID 984618.
- 1 2 3 Thompson DF, Carter JR (1993). "Drug-induced gynecomastia". Pharmacotherapy. 13 (1): 37–45. doi:10.1002/j.1875-9114.1993.tb02688.x (inactive 31 October 2021). PMID 8094898.
{{cite journal}}: CS1 maint: DOI inactive as of October 2021 (link) - 1 2 3 Shaw JC (February 1991). "Spironolactone in dermatologic therapy". J. Am. Acad. Dermatol. 24 (2 Pt 1): 236–43. doi:10.1016/0190-9622(91)70034-Y. PMID 1826112.
- 1 2 3 4 5 Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ (2017). "Oral Spironolactone for Acne Vulgaris in Adult Females: A Hybrid Systematic Review". Am J Clin Dermatol. 18 (2): 169–191. doi:10.1007/s40257-016-0245-x. PMC 5360829. PMID 28155090.
- ↑ Doggrell SA, Brown L (May 2001). "The spironolactone renaissance". Expert Opin Investig Drugs. 10 (5): 943–54. doi:10.1517/13543784.10.5.943. PMID 11322868. S2CID 39820875.
- ↑ Jashin J. Wu (18 October 2012). Comprehensive Dermatologic Drug Therapy E-Book. Elsevier Health Sciences. pp. 364–. ISBN 978-1-4557-3801-4.
Spironolactone is an aldosterone antagonist and a relatively weak antiandrogen that blocks the AR and inhibits androgen biosynthesis.
- ↑ H.J.T. Coelingh Benni; H.M. Vemer (15 December 1990). Chronic Hyperandrogenic Anovulation. CRC Press. pp. 152–. ISBN 978-1-85070-322-8.
- 1 2 Pavone-Macaluso M, de Voogt HJ, Viggiano G, Barasolo E, Lardennois B, de Pauw M, Sylvester R (September 1986). "Comparison of diethylstilbestrol, cyproterone acetate and medroxyprogesterone acetate in the treatment of advanced prostatic cancer: final analysis of a randomized phase III trial of the European Organization for Research on Treatment of Cancer Urological Group". J. Urol. 136 (3): 624–31. doi:10.1016/S0022-5347(17)44996-2. PMID 2942707.
- 1 2 Jeffrey K. Aronson (2 March 2009). Meyler's Side Effects of Cardiovascular Drugs. Elsevier. pp. 253–258. ISBN 978-0-08-093289-7.
- 1 2 Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, Speziale G, Gaudio C (2015). "Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone". Int. J. Cardiol. 200: 25–9. doi:10.1016/j.ijcard.2015.05.127. PMID 26404748.
- ↑ Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA (2004). "Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study". N. Engl. J. Med. 351 (6): 543–51. doi:10.1056/NEJMoa040135. PMID 15295047.
- 1 2 Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, Bowe WP, Graber EM, Harper JC, Kang S, Keri JE, Leyden JJ, Reynolds RV, Silverberg NB, Stein Gold LF, Tollefson MM, Weiss JS, Dolan NC, Sagan AA, Stern M, Boyer KM, Bhushan R (2016). "Guidelines of care for the management of acne vulgaris". J. Am. Acad. Dermatol. 74 (5): 945–73.e33. doi:10.1016/j.jaad.2015.12.037. PMID 26897386.
- 1 2 Plovanich M, Weng QY, Mostaghimi A (2015). "Low Usefulness of Potassium Monitoring Among Healthy Young Women Taking Spironolactone for Acne". JAMA Dermatol. 151 (9): 941–4. doi:10.1001/jamadermatol.2015.34. PMID 25796182.
- 1 2 3 Neumann F (1994). "The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research". Exp. Clin. Endocrinol. 102 (1): 1–32. doi:10.1055/s-0029-1211261. PMID 8005205.
- ↑ Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226. S2CID 28436828.
- ↑ Koch UJ, Lorenz F, Danehl K, Ericsson R, Hasan SH, Keyserlingk DV, Lübke K, Mehring M, Römmler A, Schwartz U, Hammerstein J (1976). "Continuous oral low-dosage cyproterone acetate for fertility regulation in the male? A trend analysis in 15 volunteers". Contraception. 14 (2): 117–35. doi:10.1016/0010-7824(76)90081-0. PMID 949890.
- ↑ Moltz, L.; Römmler, A.; Schwartz, U.; Hammerstein, J. (1978). "Effects of Cyproterone Acetate (CPA) on Pituitary Gonadotrophin Release and on Androgen Secretion Before and After LH-RH Double Stimulation Tests in Men". International Journal of Andrology. 1 (s2b): 713–719. doi:10.1111/j.1365-2605.1978.tb00518.x. ISSN 0105-6263.
- ↑ Wang C, Yeung KK (1980). "Use of low-dosage oral cyproterone acetate as a male contraceptive". Contraception. 21 (3): 245–72. doi:10.1016/0010-7824(80)90005-0. PMID 6771091.
- ↑ Moltz L, Römmler A, Post K, Schwartz U, Hammerstein J (April 1980). "Medium dose cyproterone acetate (CPA): effects on hormone secretion and on spermatogenesis in men". Contraception. 21 (4): 393–413. doi:10.1016/s0010-7824(80)80017-5. PMID 6771095.
- ↑ Knuth UA, Hano R, Nieschlag E (1984). "Effect of flutamide or cyproterone acetate on pituitary and testicular hormones in normal men". J. Clin. Endocrinol. Metab. 59 (5): 963–9. doi:10.1210/jcem-59-5-963. PMID 6237116.
- ↑ Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- ↑ Fung, Raymond; Hellstern-Layefsky, Miriam; Lega, Iliana (2017). "Is a lower dose of cyproterone acetate as effective at testosterone suppression in transgender women as higher doses?". International Journal of Transgenderism. 18 (2): 123–128. doi:10.1080/15532739.2017.1290566. ISSN 1553-2739. S2CID 79095497.
- ↑ Meyer G, Mayer M, Mondorf A, Fluegel AK, Herrmann E, Bojunga J (November 2019). "Safety and rapid efficacy of guideline-based gender affirming hormone therapy: an analysis of 388 individuals diagnosed with gender dysphoria". Eur. J. Endocrinol. 182 (2): 149–156. doi:10.1530/EJE-19-0463. PMID 31751300. S2CID 208229129.
- ↑ Pucci E, Petraglia F (December 1997). "Treatment of androgen excess in females: yesterday, today and tomorrow". Gynecol. Endocrinol. 11 (6): 411–33. doi:10.3109/09513599709152569. PMID 9476091.
- ↑ Pharmacology of the Skin II: Methods, Absorption, Metabolism and Toxicity, Drugs and Diseases. Springer Science & Business Media. 6 December 2012. pp. 474, 489. ISBN 978-3-642-74054-1.
- ↑ Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A (2004). "Hepatotoxicity induced by antiandrogens: a review of the literature". Urol. Int. 73 (4): 289–95. doi:10.1159/000081585. PMID 15604569. S2CID 24799765.
- ↑ Hammerstein, J. (1990). "Antiandrogens: Clinical Aspects". Hair and Hair Diseases. pp. 827–886. doi:10.1007/978-3-642-74612-3_35. ISBN 978-3-642-74614-7.
- ↑ Lothstein, Leslie M. (1996). "Antiandrogen treatment for sexual disorders: Guidelines for establishing a standard of care". Sexual Addiction & Compulsivity. 3 (4): 313–331. doi:10.1080/10720169608400122. ISSN 1072-0162.
- ↑ Dangerous Sex Offenders: A Task Force Report of the American Psychiatric Association. American Psychiatric Pub. 1999. pp. 112–144. ISBN 978-0-89042-280-9.
- ↑ Kravitz HM, Haywood TW, Kelly J, Liles S, Cavanaugh JL (1996). "Medroxyprogesterone and paraphiles: do testosterone levels matter?". Bull Am Acad Psychiatry Law. 24 (1): 73–83. PMID 8891323.
- ↑ Novak E, Hendrix JW, Chen TT, Seckman CE, Royer GL, Pochi PE (October 1980). "Sebum production and plasma testosterone levels in man after high-dose medroxyprogesterone acetate treatment and androgen administration". Acta Endocrinol. 95 (2): 265–70. doi:10.1530/acta.0.0950265. PMID 6449127.
- ↑ Kirschner MA, Schneider G (February 1972). "Suppression of the pituitary-Leydig cell axis and sebum production in normal men by medroxyprogesterone acetate (provera)". Acta Endocrinol. 69 (2): 385–93. doi:10.1530/acta.0.0690385. PMID 5066846.
- ↑ Kemppainen JA, Langley E, Wong CI, Bobseine K, Kelce WR, Wilson EM (March 1999). "Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone". Mol. Endocrinol. 13 (3): 440–54. doi:10.1210/mend.13.3.0255. PMID 10077001.
- ↑ Westhoff C (August 2003). "Depot-medroxyprogesterone acetate injection (Depo-Provera): a highly effective contraceptive option with proven long-term safety". Contraception. 68 (2): 75–87. doi:10.1016/S0010-7824(03)00136-7. PMID 12954518.
- ↑ Nieschlag E (November 2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
- ↑ Nieschlag E, Zitzmann M, Kamischke A (November 2003). "Use of progestins in male contraception". Steroids. 68 (10–13): 965–72. doi:10.1016/S0039-128X(03)00135-1. PMID 14667989. S2CID 22458746.
- ↑ Wu FC, Balasubramanian R, Mulders TM, Coelingh-Bennink HJ (January 1999). "Oral progestogen combined with testosterone as a potential male contraceptive: additive effects between desogestrel and testosterone enanthate in suppression of spermatogenesis, pituitary-testicular axis, and lipid metabolism". J. Clin. Endocrinol. Metab. 84 (1): 112–22. doi:10.1210/jcem.84.1.5412. PMID 9920070.
- ↑ Kumamoto Y, Yamaguchi Y, Sato Y, Suzuki R, Tanda H, Kato S, Mori K, Matsumoto H, Maki A, Kadono M (February 1990). "[Effects of anti-androgens on sexual function. Double-blind comparative studies on allylestrenol and chlormadinone acetate Part I: Nocturnal penile tumescence monitoring]". Hinyokika Kiyo (in Japanese). 36 (2): 213–26. PMID 1693037.
- ↑ Geller J, Albert J, Geller S (1982). "Acute therapy with megestrol acetate decreases nuclear and cytosol androgen receptors in human BPH tissue". The Prostate. 3 (1): 11–5. doi:10.1002/pros.2990030103. PMID 6176985. S2CID 23541558.
- ↑ Sander S, Nissen-Meyer R, Aakvaag A (1978). "On gestagen treatment of advanced prostatic carcinoma". Scand. J. Urol. Nephrol. 12 (2): 119–21. doi:10.3109/00365597809179977. PMID 694436.
- ↑ Hinman, Frank, Jr. (1983). Benign Prostatic Hypertrophy. Springer Science & Business Media. pp. 259, 266, 272. ISBN 978-1-4612-5476-8.
- ↑ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- ↑ A. Hughes; S. H. Hasan; G. W. Oertel; H. E. Voss, F. Bahner, F. Neumann, H. Steinbeck, K.-J. Gräf, J. Brotherton, H. J. Horn, R. K. Wagner (27 November 2013). Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer Science & Business Media. pp. 490–491. ISBN 978-3-642-80859-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Wenderoth, U. K.; Jacobi, G. H. (1983). "Gonadotropin-releasing hormone analogues for palliation of carcinoma of the prostate". World Journal of Urology. 1 (1): 40–48. doi:10.1007/BF00326861. ISSN 0724-4983. S2CID 23447326.
- ↑ Schröder, Fritz H.; Radlmaier, Albert (2009). "Steroidal Antiandrogens". In V. Craig Jordan; Barrington J. A. Furr (eds.). Hormone Therapy in Breast and Prostate Cancer. Humana Press. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.
CPA, as mentioned earlier, leads to an incomplete suppression of plasma testosterone levels, which decrease by about 70% and remain at about three times castration values. [Rennie et al.] found that the combination of CPA with an extremely low dose (0.1 mg/d) of DES led to a very effective withdrawal of androgens in terms of plasma testosterone and tissue dihydrotestosterone. [...] this regimen combines the testosterone-reducing effects of two compounds, therefore, only small amounts of estrogen are required to bring down plasma testosterone to approximately castrate levels.
- ↑ Melamed AJ (March 1987). "Current concepts in the treatment of prostate cancer". Drug Intell Clin Pharm. 21 (3): 247–54. doi:10.1177/106002808702100302. PMID 3552544. S2CID 7482144.
[Megestrol acetate] produces a transient reduction in plasma testosterone to levels somewhat higher than those in castrated men. When used in a dose of 40 mg tid, in combination with estradiol 0.5–1.5 mg/d, it acts synergistically to suppress pituitary gonadotropins and maintain plasma testosterone at castration levels for periods up to one year.
- 1 2 3 4 5 6 Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1286–1288. ISBN 978-0-7817-6879-5.
- 1 2 3 Giorgetti R, di Muzio M, Giorgetti A, Girolami D, Borgia L, Tagliabracci A (March 2017). "Flutamide-induced hepatotoxicity: ethical and scientific issues" (PDF). Eur Rev Med Pharmacol Sci. 21 (1 Suppl): 69–77. PMID 28379593.
- 1 2 Erem C (2013). "Update on idiopathic hirsutism: diagnosis and treatment". Acta Clin Belg. 68 (4): 268–74. doi:10.2143/ACB.3267. PMID 24455796. S2CID 39120534.
- 1 2 Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A (March 2018). "Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial". J. Clin. Endocrinol. Metab. 103 (3): 824–838. doi:10.1210/jc.2017-01186. PMID 29211888. S2CID 3784055.
- 1 2 3 William D. Figg; Cindy H. Chau; Eric J. Small (14 September 2010). Drug Management of Prostate Cancer. Springer Science & Business Media. pp. 71–72. ISBN 978-1-60327-829-4.
- ↑ Caubet JF, Tosteson TD, Dong EW, Naylon EM, Whiting GW, Ernstoff MS, Ross SD (January 1997). "Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens". Urology. 49 (1): 71–8. doi:10.1016/S0090-4295(96)00325-1. PMID 9000189.
- ↑ Bruce A. Chabner; Dan L. Longo (8 November 2010). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. pp. 680–. ISBN 978-1-60547-431-1.
- ↑ Neyman, A; Fuqua, JS; Eugster, EA (December 2017). "Bicalutamide as an Androgen Blocker with Secondary Effect of Promoting Feminization in Male to Female (MTF) Transgender Adolescents". Hormone Research in Paediatrics. 88: 1–628. doi:10.1159/000481424. PMID 28968603.
- ↑ Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, Denis L, Iversen P, Eisenberger MA, Labrie F (May 2018). "Androgen Receptor-Targeted Treatments for Prostate Cancer: 35 Years' Progress with Antiandrogens". J. Urol. 200 (5): 956–966. doi:10.1016/j.juro.2018.04.083. PMID 29730201. S2CID 19162538.
- ↑ Ito Y, Sadar MD (2018). "Enzalutamide and blocking androgen receptor in advanced prostate cancer: lessons learnt from the history of drug development of antiandrogens". Res Rep Urol. 10: 23–32. doi:10.2147/RRU.S157116. PMC 5818862. PMID 29497605.
- 1 2 Ricci F, Buzzatti G, Rubagotti A, Boccardo F (November 2014). "Safety of antiandrogen therapy for treating prostate cancer". Expert Opin Drug Saf. 13 (11): 1483–99. doi:10.1517/14740338.2014.966686. PMID 25270521. S2CID 207488100.
- ↑ Lutz Moser (1 January 2008). Controversies in the Treatment of Prostate Cancer. Karger Medical and Scientific Publishers. pp. 41–. ISBN 978-3-8055-8524-8.
- 1 2 Prostate Cancer. Demos Medical Publishing. 20 December 2011. pp. 460, 504. ISBN 978-1-935281-91-7.
- ↑ Chang S (10 March 2010), Bicalutamide BPCA Drug Use Review in the Pediatric Population (PDF), U.S. Department of Health and Human Service, archived (PDF) from the original on 24 October 2016, retrieved 20 July 2016
- ↑ Kolvenbag GJ, Blackledge GR (January 1996). "Worldwide activity and safety of bicalutamide: a summary review". Urology. 47 (1A Suppl): 70–9, discussion 80–4. doi:10.1016/S0090-4295(96)80012-4. PMID 8560681.
- ↑ Vogelzang NJ (September 2012). "Enzalutamide--a major advance in the treatment of metastatic prostate cancer". N. Engl. J. Med. 367 (13): 1256–7. doi:10.1056/NEJMe1209041. PMID 23013078.
- ↑ J. Ramon; L.J. Denis (5 June 2007). Prostate Cancer. Springer Science & Business Media. pp. 256–. ISBN 978-3-540-40901-4.
- ↑ Gretarsdottir, Helga M.; Bjornsdottir, Elin; Bjornsson, Einar S. (2018). "Bicalutamide-Associated Acute Liver Injury and Migratory Arthralgia: A Rare but Clinically Important Adverse Effect". Case Reports in Gastroenterology. 12 (2): 266–270. doi:10.1159/000485175. ISSN 1662-0631. S2CID 81661015.
- ↑ Gao Y, Maurer T, Mirmirani P (January 2018). "Understanding and Addressing Hair Disorders in Transgender Individuals". Am J Clin Dermatol. 19 (4): 517–527. doi:10.1007/s40257-018-0343-z. PMID 29352423. S2CID 6467968.
Non-steroidal antiandrogens include flutamide, nilutamide, and bicalutamide, which do not lower androgen levels and may be favorable for individuals who want to preserve sex drive and fertility [9].
- ↑ Iversen P, Melezinek I, Schmidt A (Jan 2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992. S2CID 28215804.
- ↑ Morgante, E; Gradini, R; Realacci, M; Sale, P; D'eramo, G; Perrone, G A; Cardillo, M R; Petrangeli, E; Russo, Ma; Di Silverio, F (2001). "Effects of long-term treatment with the anti-androgen bicalutamide on human testis: an ultrastructural and morphometric study". Histopathology. 38 (3): 195–201. doi:10.1046/j.1365-2559.2001.01077.x. ISSN 0309-0167. PMID 11260298. S2CID 36892099.
- ↑ Jones CA, Reiter L, Greenblatt E (2016). "Fertility preservation in transgender patients". International Journal of Transgenderism. 17 (2): 76–82. doi:10.1080/15532739.2016.1153992. ISSN 1553-2739. S2CID 58849546.
Traditionally, patients have been advised to cryopreserve sperm prior to starting cross-sex hormone therapy as there is a potential for a decline in sperm motility with high-dose estrogen therapy over time (Lubbert et al., 1992). However, this decline in fertility due to estrogen therapy is controversial due to limited studies.
- ↑ Payne AH, Hardy MP (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 422–431. ISBN 978-1-59745-453-7.
Estrogens are highly efficient inhibitors of the hypothalamic-hypophyseal-testicular axis (212–214). Aside from their negative feedback action at the level of the hypothalamus and pituitary, direct inhibitory effects on the testis are likely (215,216). [...] The histology of the testes [with estrogen treatment] showed disorganization of the seminiferous tubules, vacuolization and absence of lumen, and compartmentalization of spermatogenesis.
- 1 2 Salam MA (2003). Principles & Practice of Urology: A Comprehensive Text. Universal-Publishers. pp. 684–. ISBN 978-1-58112-412-5.
Estrogens act primarily through negative feedback at the hypothalamic-pituitary level to reduce LH secretion and testicular androgen synthesis. [...] Interestingly, if the treatment with estrogens is discontinued after 3 yr. of uninterrupted exposure, serum testosterone may remain at castration levels for up to another 3 yr. This prolonged suppression is thought to result from a direct effect of estrogens on the Leydig cells.
- 1 2 3 Cox RL, Crawford ED (December 1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/S0022-5347(01)66670-9. PMID 7500443.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Engel JB, Schally AV (February 2007). "Drug Insight: clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone". Nat Clin Pract Endocrinol Metab. 3 (2): 157–67. doi:10.1038/ncpendmet0399. PMID 17237842. S2CID 19745821.
- 1 2 3 4 Shlomo Melmed (1 January 2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 154, 621, 711. ISBN 978-0-323-29738-7.
- ↑ Timothy L. Ratliff; William J. Catalona (6 December 2012). Genitourinary Cancer: Basic and Clinical Aspects. Springer Science & Business Media. pp. 158–. ISBN 978-1-4613-2033-3.
- ↑ Ezzati M, Carr BR (January 2015). "Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain". Womens Health (Lond). 11 (1): 19–28. doi:10.2217/whe.14.68. PMID 25581052. S2CID 7516507.
- ↑ Conn PM, Crowley WF (January 1991). "Gonadotropin-releasing hormone and its analogues". N. Engl. J. Med. 324 (2): 93–103. doi:10.1056/NEJM199101103240205. PMID 1984190.
- ↑ Jerome F. Strauss; Jerome F. Strauss, III; Robert L. Barbieri (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 272–. ISBN 978-1-4557-2758-2.
- 1 2 3 Krakowsky Y, Morgentaler A (July 2017). "Risk of Testosterone Flare in the Era of the Saturation Model: One More Historical Myth". Eur Urol Focus. 5 (1): 81–89. doi:10.1016/j.euf.2017.06.008. PMID 28753828. S2CID 10011200.
- 1 2 Thompson IM (2001). "Flare Associated with LHRH-Agonist Therapy". Rev Urol. 3 Suppl 3: S10–4. PMC 1476081. PMID 16986003.
- ↑ Scaletscky R, Smith JA (April 1993). "Disease flare with gonadotrophin-releasing hormone (GnRH) analogues. How serious is it?". Drug Saf. 8 (4): 265–70. doi:10.2165/00002018-199308040-00001. PMID 8481213. S2CID 36964191.
- 1 2 3 4 J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2009, 2207, 2479. ISBN 978-0-323-32195-2.
- ↑ Louis J Denis; Keith Griffiths; Amir V Kaisary; Gerald P Murphy (1 March 1999). Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. pp. 308–. ISBN 978-1-85317-422-3.
- ↑ Reilly DR, Delva NJ, Hudson RW (August 2000). "Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia". Can J Psychiatry. 45 (6): 559–63. doi:10.1177/070674370004500608. PMID 10986575. S2CID 27710792.
[...] estrogen or antiandrogen treatment prior to the first leuprolide injection may reduce [the risk of symptoms caused by the testosterone "flare" at the initiation of treatment] (16).
- 1 2 3 4 Dittrich R, Binder H, Cupisti S, Hoffmann I, Beckmann MW, Mueller A (December 2005). "Endocrine treatment of male-to-female transsexuals using gonadotropin-releasing hormone agonist". Exp. Clin. Endocrinol. Diabetes. 113 (10): 586–92. doi:10.1055/s-2005-865900. PMID 16320157.
- 1 2 3 Loren S Schechter; Bauback Safa (23 June 2018). Gender Confirmation Surgery, An Issue of Clinics in Plastic Surgery, E-Book. Elsevier Health Sciences. pp. 314–. ISBN 978-0-323-61075-9.
- ↑ Emans SJ, Laufer MR (5 January 2012). Emans, Laufer, Goldstein's Pediatric and Adolescent Gynecology. Lippincott Williams & Wilkins. pp. 365–. ISBN 978-1-4511-5406-1. Archived from the original on 16 May 2016.
Therapy with GnRH analogs is expensive and requires intramuscular injections of depot formulations, the insert of a subcutaneous implant yearly, or, much less commonly, daily subcutaneous injections.
- ↑ Hillard PJ (29 March 2013). Practical Pediatric and Adolescent Gynecology. John Wiley & Sons. pp. 182–. ISBN 978-1-118-53857-9.
Treatment is expensive, with costs typically in the range of $10,000–$15,000 per year.
- ↑ Everett E. Vokes; Harvey M. Golomb (28 June 2011). Oncologic Therapies. Springer Science & Business Media. pp. 493–. ISBN 978-3-642-55780-4.
- 1 2 T'Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V (October 2018). "Endocrinology of Transgender Medicine". Endocrine Reviews. 40 (1): 97–117. doi:10.1210/er.2018-00011. PMID 30307546.
- ↑ Cone, Allen (25 July 2018). "FDA approves drug to control endometriosis pain". UPI. Retrieved 31 July 2018.
- 1 2 3 4 5 6 7 8 9 10 11 12 Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA (June 2017). "Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels". Endocr. Rev. 38 (3): 220–254. doi:10.1210/er.2016-1067. PMC 6459338. PMID 28472278.
- 1 2 3 4 5 6 7 8 9 10 Marchetti PM, Barth JH (March 2013). "Clinical biochemistry of dihydrotestosterone". Ann. Clin. Biochem. 50 (Pt 2): 95–107. doi:10.1258/acb.2012.012159. PMID 23431485. S2CID 8325257.
- ↑ Mozayani A, Raymon L (18 September 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
- 1 2 Marks LS (2004). "5α-reductase: history and clinical importance". Rev Urol. 6 Suppl 9: S11–21. PMC 1472916. PMID 16985920.
- ↑ Bhasin S (13 February 1996). Pharmacology, Biology, and Clinical Applications of Androgens: Current Status and Future Prospects. John Wiley & Sons. pp. 72–. ISBN 978-0-471-13320-9.
- ↑ Jin Y, Penning TM (2001). "Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism". Best Pract. Res. Clin. Endocrinol. Metab. 15 (1): 79–94. doi:10.1053/beem.2001.0120. PMID 11469812.
- ↑ Horton R (1992). "Dihydrotestosterone is a peripheral paracrine hormone". J. Androl. 13 (1): 23–7. doi:10.1002/j.1939-4640.1992.tb01621.x. PMID 1551803.
- ↑ Wilson JD (1996). "Role of dihydrotestosterone in androgen action". Prostate Suppl. 6 (S6): 88–92. doi:10.1002/(SICI)1097-0045(1996)6+<88::AID-PROS17>3.0.CO;2-N. PMID 8630237.
- ↑ Okeigwe I, Kuohung W (December 2014). "5-Alpha reductase deficiency: a 40-year retrospective review". Curr Opin Endocrinol Diabetes Obes. 21 (6): 483–7. doi:10.1097/MED.0000000000000116. PMID 25321150. S2CID 1093345.
- ↑ Imperato-McGinley J, Zhu YS (December 2002). "Androgens and male physiology the syndrome of 5alpha-reductase-2 deficiency". Mol. Cell. Endocrinol. 198 (1–2): 51–9. doi:10.1016/S0303-7207(02)00368-4. PMID 12573814. S2CID 54356569.
- ↑ Liang, Jennifer J.; Rasmusson, Ann M. (2018). "Overview of the Molecular Steps in Steroidogenesis of the GABAergic Neurosteroids Allopregnanolone and Pregnanolone". Chronic Stress. 2: 247054701881855. doi:10.1177/2470547018818555. ISSN 2470-5470. PMC 7219929. PMID 32440589.
- 1 2 3 Traish AM, Mulgaonkar A, Giordano N (June 2014). "The dark side of 5α-reductase inhibitors' therapy: sexual dysfunction, high Gleason grade prostate cancer and depression". Korean J Urol. 55 (6): 367–79. doi:10.4111/kju.2014.55.6.367. PMC 4064044. PMID 24955220.
- 1 2 3 Bartsch G, Rittmaster RS, Klocker H (April 2000). "Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia". Eur. Urol. 37 (4): 367–80. doi:10.1159/000020181. PMID 10765065. S2CID 25793400.
- 1 2 Yamana K, Labrie F, Luu-The V (August 2010). "Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride". Horm Mol Biol Clin Investig. 2 (3): 293–9. doi:10.1515/HMBCI.2010.035. PMID 25961201. S2CID 28841145.
- ↑ Traish AM, Krakowsky Y, Doros G, Morgentaler A (August 2018). "Do 5α-Reductase Inhibitors Raise Circulating Serum Testosterone Levels? A Comprehensive Review and Meta-Analysis to Explaining Paradoxical Results". Sex Med Rev. 7 (1): 95–114. doi:10.1016/j.sxmr.2018.06.002. PMID 30098986. S2CID 51968365.
- ↑ Azzouni F, Mohler J (September 2012). "Role of 5α-reductase inhibitors in benign prostatic diseases". Prostate Cancer Prostatic Dis. 15 (3): 222–30. doi:10.1038/pcan.2012.1. PMID 22333687. S2CID 205537645.
- 1 2 Yim E, Nole KL, Tosti A (December 2014). "5α-Reductase inhibitors in androgenetic alopecia". Curr Opin Endocrinol Diabetes Obes. 21 (6): 493–8. doi:10.1097/MED.0000000000000112. PMID 25268732. S2CID 30008068.
- 1 2 Arif T, Dorjay K, Adil M, Sami M (2017). "Dutasteride in Androgenetic Alopecia: An Update". Curr Clin Pharmacol. 12 (1): 31–35. doi:10.2174/1574884712666170310111125. PMID 28294070.
- 1 2 3 Stout SM, Stumpf JL (June 2010). "Finasteride treatment of hair loss in women". Ann Pharmacother. 44 (6): 1090–7. doi:10.1345/aph.1M591. PMID 20442354. S2CID 207263793.
- ↑ Varothai S, Bergfeld WF (July 2014). "Androgenetic alopecia: an evidence-based treatment update". Am J Clin Dermatol. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508. S2CID 31245042.
- ↑ Ulrike Blume-Peytavi; David A. Whiting; Ralph M. Trüeb (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. pp. 182, 369. ISBN 978-3-540-46911-7.
- ↑ Jerry Shapiro; Nina Otberg (17 April 2015). Hair Loss and Restoration, Second Edition. CRC Press. pp. 39–40. ISBN 978-1-4822-3199-1.
- ↑ Ralph M. Trüeb; Won-Soo Lee (13 February 2014). Male Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 91–. ISBN 978-3-319-03233-7.
- 1 2 Reddy DS, Estes WA (July 2016). "Clinical Potential of Neurosteroids for CNS Disorders". Trends Pharmacol. Sci. 37 (7): 543–561. doi:10.1016/j.tips.2016.04.003. PMC 5310676. PMID 27156439.
- 1 2 Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, Cintron D, Thompson KD, Khine KK, Schmidt PJ (March 2016). "5α-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder". Neuropsychopharmacology. 41 (4): 1093–102. doi:10.1038/npp.2015.246. PMC 4748434. PMID 26272051.
- 1 2 Knezevich EL, Viereck LK, Drincic AT (January 2012). "Medical management of adult transsexual persons". Pharmacotherapy. 32 (1): 54–66. doi:10.1002/PHAR.1006. PMID 22392828. S2CID 12853220.
- ↑ Fabris B, Bernardi S, Trombetta C (March 2015). "Cross-sex hormone therapy for gender dysphoria". J. Endocrinol. Invest. 38 (3): 269–82. doi:10.1007/s40618-014-0186-2. PMID 25403429. S2CID 207503049.
- 1 2 3 4 5 6 7 Levy A, Crown A, Reid R (October 2003). "Endocrine intervention for transsexuals". Clin. Endocrinol. (Oxf). 59 (4): 409–18. doi:10.1046/j.1365-2265.2003.01821.x. PMID 14510900. S2CID 24493388.
- 1 2 3 Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS (July 2016). "Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review". J Clin Aesthet Dermatol. 9 (7): 56–62. PMC 5023004. PMID 27672412.
- 1 2 3 Trost L, Saitz TR, Hellstrom WJ (May 2013). "Side Effects of 5-Alpha Reductase Inhibitors: A Comprehensive Review". Sex Med Rev. 1 (1): 24–41. doi:10.1002/smrj.3. PMID 27784557.
- 1 2 Liu L, Zhao S, Li F, Li E, Kang R, Luo L, Luo J, Wan S, Zhao Z (September 2016). "Effect of 5α-Reductase Inhibitors on Sexual Function: A Meta-Analysis and Systematic Review of Randomized Controlled Trials". J Sex Med. 13 (9): 1297–1310. doi:10.1016/j.jsxm.2016.07.006. PMID 27475241.
- 1 2 3 Lee JY, Cho KS (May 2018). "Effects of 5-alpha reductase inhibitors: new insights on benefits and harms". Curr Opin Urol. 28 (3): 288–293. doi:10.1097/MOU.0000000000000497. PMID 29528971. S2CID 4587434.
- 1 2 Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML (March 2011). "Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients". J Sex Med. 8 (3): 872–84. doi:10.1111/j.1743-6109.2010.02157.x. PMID 21176115.
- 1 2 Traish, Abdulmaged M. (2018). "The Post-finasteride Syndrome: Clinical Manifestation of Drug-Induced Epigenetics Due to Endocrine Disruption". Current Sexual Health Reports. 10 (3): 88–103. doi:10.1007/s11930-018-0161-6. ISSN 1548-3584. S2CID 81560714.
- ↑ Malde S, Cartwright R, Tikkinen KA (January 2018). "What's New in Epidemiology?". Eur Urol Focus. 4 (1): 11–13. doi:10.1016/j.euf.2018.02.003. PMID 29449167.
- ↑ Kuhl, Herbert; Wiegratz, Inka (2017). "Das Post-Finasterid-Syndrom" [The Post-Finasteride Syndrome]. Gynäkologische Endokrinologie. 15 (2): 153–163. doi:10.1007/s10304-017-0126-2. ISSN 1610-2894. S2CID 207071180.
- ↑ Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M (September 2015). "Adverse effects of 5α-reductase inhibitors: What do we know, don't know, and need to know?". Rev Endocr Metab Disord. 16 (3): 177–98. doi:10.1007/s11154-015-9319-y. PMID 26296373. S2CID 25002351.
- ↑ Trüeb RM (June 2017). "Discriminating in favour of or against men with increased risk of finasteride-related side effects?". Exp. Dermatol. 26 (6): 527–528. doi:10.1111/exd.13155. PMID 27489125. S2CID 36236057.
[...] caution is recommended while prescribing oral finasteride to male-to-female transsexuals, as the drug has been associated with inducing depression, anxiety and suicidal ideation, symptoms that are particularly common in patients with gender dysphoria, who are already at a high risk.[9]
- ↑ Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1397–1399. ISBN 978-1-60913-345-0.
- 1 2 3 4 5 6 7 Macias, Hector; Hinck, Lindsay (2012). "Mammary gland development". Wiley Interdisciplinary Reviews: Developmental Biology. 1 (4): 533–557. doi:10.1002/wdev.35. ISSN 1759-7684. PMC 3404495. PMID 22844349.
- 1 2 3 4 5 6 Sun, Susie X.; Bostanci, Zeynep; Kass, Rena B.; Mancino, Anne T.; Rosenbloom, Arlan L.; Klimberg, V. Suzanne; Bland, Kirby I. (2018). "Breast Physiology". The Breast. pp. 37–56.e6. doi:10.1016/B978-0-323-35955-9.00003-9. ISBN 9780323359559.
- 1 2 3 4 5 6 7 8 Wierckx K, Gooren L, T'Sjoen G (2014). "Clinical review: Breast development in trans women receiving cross-sex hormones". J Sex Med. 11 (5): 1240–7. doi:10.1111/jsm.12487. PMID 24618412.
- ↑ Cox DB, Kent JC, Casey TM, Owens RA, Hartmann PE (March 1999). "Breast growth and the urinary excretion of lactose during human pregnancy and early lactation: endocrine relationships". Exp. Physiol. 84 (2): 421–34. doi:10.1017/S0958067099018072. PMID 10226182.
- 1 2 3 Wiegratz I, Kuhl H (August 2004). "Progestogen therapies: differences in clinical effects?". Trends Endocrinol. Metab. 15 (6): 277–85. doi:10.1016/j.tem.2004.06.006. PMID 15358281. S2CID 35891204.
- ↑ Mary C. Brucker; Tekoa L. King (8 September 2015). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 368–. ISBN 978-1-284-05748-5.
- 1 2 3 4 5 6 7 Fabris B, Bernardi S, Trombetta C (March 2015). "Cross-sex hormone therapy for gender dysphoria". J. Endocrinol. Invest. 38 (3): 269–82. doi:10.1007/s40618-014-0186-2. PMID 25403429. S2CID 207503049.
- 1 2 Ilan H. Meyer; Mary E. Northridge (12 March 2007). The Health of Sexual Minorities: Public Health Perspectives on Lesbian, Gay, Bisexual and Transgender Populations. Springer. pp. 476–. ISBN 978-0-387-31334-4.
- ↑ Gianna E. Israel; Donald E. Tarver; Joy Diane Shaffer (1 March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 58–. ISBN 978-1-56639-852-7.
- ↑ Richard Ekins; Dave King (23 October 2006). The Transgender Phenomenon. SAGE Publications. pp. 48–. ISBN 978-1-84787-726-0.
- 1 2 Kronawitter D, Gooren LJ, Zollver H, Oppelt PG, Beckmann MW, Dittrich R, Mueller A (August 2009). "Effects of transdermal testosterone or oral dydrogesterone on hypoactive sexual desire disorder in transsexual women: results of a pilot study". Eur. J. Endocrinol. 161 (2): 363–8. doi:10.1530/EJE-09-0265. PMID 19497984.
- ↑ Majumder A, Sanyal D (2017). "Outcome and preferences in male-to-female subjects with gender dysphoria: Experience from Eastern India". Indian J Endocrinol Metab. 21 (1): 21–25. doi:10.4103/2230-8210.196000. PMC 5240066. PMID 28217493.
- 1 2 Meyer WJ, Webb A, Stuart CA, Finkelstein JW, Lawrence B, Walker PA (April 1986). "Physical and hormonal evaluation of transsexual patients: a longitudinal study". Archives of Sexual Behavior. 15 (2): 121–38. doi:10.1007/bf01542220. PMID 3013122. S2CID 42786642.
- ↑ Daniel R. Mishell; Val Davajan (1979). Reproductive endocrinology, infertility, and contraception. F. A. Davis Co. p. 224. ISBN 978-0-8036-6235-3.
It has been suggested that progestins be added during the last week of each cycle of estrogen therapy in order to develop more rounded breasts rather than the conical breasts many of these patients develop, but we have been unable to detect any difference in breast contour with or without progestins.
- ↑ Morris JM (June 1953). "The syndrome of testicular feminization in male pseudohermaphrodites". Am. J. Obstet. Gynecol. 65 (6): 1192–1211. doi:10.1016/0002-9378(53)90359-7. PMID 13057950.
- ↑ Lorincz AM, Sukumar S (2006). "Molecular links between obesity and breast cancer". Endocrine-Related Cancer. 13 (2): 279–92. doi:10.1677/erc.1.00729. PMID 16728564.
Adipocytes make up the bulk of the human breast, with epithelial cells accounting for only approximately 10% of human breast volume.
- ↑ Howard BA, Gusterson BA (2000). "Human breast development". Journal of Mammary Gland Biology and Neoplasia. 5 (2): 119–37. doi:10.1023/A:1026487120779. PMID 11149569. S2CID 10819224.
In the stroma, there is an increase in the amount of fibrous and fatty tissue, with the adult nonlactating breast consisting of 80% or more of stroma.
- ↑ Sperling MA (10 April 2014). Pediatric Endocrinology. Elsevier Health Sciences. pp. 598–. ISBN 978-1-4557-5973-6.
Estrogen stimulates the nipples to grow, mammary terminal duct branching to progress to the stage at which ductules are formed, and fatty stromal growth to increase until it constitutes about 85% of the mass of the breast. [...] Lobulation appears around menarche, when multiple blind saccular buds form by branching of the terminal ducts. These effects are due to the presence of progesterone. [...] Full alveolar development normally only occurs during pregnancy under the influence of additional progesterone and prolactin.
- ↑ Hagisawa S, Shimura N, Arisaka O (2012). "Effect of excess estrogen on breast and external genitalia development in growth hormone deficiency". Journal of Pediatric and Adolescent Gynecology. 25 (3): e61–3. doi:10.1016/j.jpag.2011.11.005. PMID 22206682.
Estrogen stimulates growth of the nipples, progression of mammary duct branching to the stage at which ductiles are formed, and fatty stromal growth until it constitutes about 85% of the mass of the breast.
- 1 2 Lee-Ellen C. Copstead-Kirkhorn; Jacquelyn L. Banasik (25 June 2014). Pathophysiology - E-Book. Elsevier Health Sciences. pp. 660–. ISBN 978-0-323-29317-4.
Throughout the reproductive years, some women note swelling of the breast around the latter part of each menstrual cycle before the onset of menstruation. The water retention and subsequent swelling of breast tissue during this phase of the menstrual cycle are thought to be due to high levels of circulating progesterone stimulating the secretory cells of the breast.12
- 1 2 Farage MA, Neill S, MacLean AB (2009). "Physiological changes associated with the menstrual cycle: a review". Obstet Gynecol Surv. 64 (1): 58–72. doi:10.1097/OGX.0b013e3181932a37. PMID 19099613. S2CID 22293838.
- ↑ Gompel A (April 2012). "Micronized progesterone and its impact on the endometrium and breast vs. progestogens". Climacteric. 15 Suppl 1: 18–25. doi:10.3109/13697137.2012.669584. PMID 22432812. S2CID 17700754.
- ↑ Cline JM, Wood CE (December 2008). "The Mammary Glands of Macaques". Toxicol Pathol. 36 (7): 134s–141s. doi:10.1177/0192623308327411. PMC 3070964. PMID 21475638.
- ↑ Pasqualini JR (2007). "Progestins and breast cancer". Gynecol. Endocrinol. 23 Suppl 1: 32–41. doi:10.1080/09513590701585003. PMID 17943537. S2CID 46634314.
- ↑ Pasqualini JR (2009). "Breast cancer and steroid metabolizing enzymes: the role of progestogens". Maturitas. 65 Suppl 1: S17–21. doi:10.1016/j.maturitas.2009.11.006. PMID 19962254.
- ↑ Schindler AE (February 2011). "Dydrogesterone and other progestins in benign breast disease: an overview". Arch. Gynecol. Obstet. 283 (2): 369–71. doi:10.1007/s00404-010-1456-7. PMID 20383772. S2CID 9125889.
- ↑ Winkler UH, Schindler AE, Brinkmann US, Ebert C, Oberhoff C (December 2001). "Cyclic progestin therapy for the management of mastopathy and mastodynia". Gynecol. Endocrinol. 15 Suppl 6: 37–43. doi:10.1080/gye.15.s6.37.43. PMID 12227885. S2CID 27589741.
- 1 2 3 4 Ruan X, Mueck AO (November 2014). "Systemic progesterone therapy--oral, vaginal, injections and even transdermal?". Maturitas. 79 (3): 248–55. doi:10.1016/j.maturitas.2014.07.009. PMID 25113944.
- ↑ Bińkowska, Małgorzata; Woroń, Jarosław (2015). "Progestogens in menopausal hormone therapy". Menopausal Review. 14 (2): 134–143. doi:10.5114/pm.2015.52154. ISSN 1643-8876. PMC 4498031. PMID 26327902.
- ↑ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889–. ISBN 978-0-7817-1750-2.
- ↑ Sanjay Rajagopalan; Debabrata Mukherjee; Emile R. Mohler (2005). Manual of Vascular Diseases. Lippincott Williams & Wilkins. pp. 1–. ISBN 978-0-7817-4499-7.
- 1 2 3 Foss GL (March 1958). "Disturbances of lactation". Clin Obstet Gynecol. 1 (1): 245–54. doi:10.1097/00003081-195803000-00021. PMID 13573669. S2CID 42825519.
Experimentally I have been able to induce lactogenesis in a male transvestite whose testes had been removed some years before and whose breasts had been well developed over a long period with stilbestrol and ethisterone.9 In July, 1955, 600 mg. of estradiol was implanted subcutaneously and weekly injections of 50 mg. of progesterone were given for four months. For the next month daily injections of 10 mg. estradiol dipropionate and 50 mg. progesterone were given. These injections were continued for another month, increasing progesterone to 100 mg. daily. Both hormones were then withdrawn, and daily injections of increasing doses of prolactin and somatotropin were given for four days; at the same time, the patient used a breast bump four times daily for 5 minutes on both sides. During this time the mammary veins were visibly enlarged and on the sixth and seventh days 1 to 2 cc. of milky fluid was collected.
- 1 2 3 Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW (January 2000). "Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men". The American Journal of Surgical Pathology. 24 (1): 74–80. doi:10.1097/00000478-200001000-00009. PMID 10632490.
- ↑ Lawrence, Anne A. (2007). "Transgender Health Concerns". The Health of Sexual Minorities: 473–505. doi:10.1007/978-0-387-31334-4_19. ISBN 978-0-387-28871-0.
- ↑ Paul Peter Rosen (2009). Rosen's Breast Pathology. Lippincott Williams & Wilkins. pp. 31–. ISBN 978-0-7817-7137-5.
- ↑ Worsley R, Santoro N, Miller KK, Parish SJ, Davis SR (March 2016). "Hormones and Female Sexual Dysfunction: Beyond Estrogens and Androgens--Findings from the Fourth International Consultation on Sexual Medicine". J Sex Med. 13 (3): 283–90. doi:10.1016/j.jsxm.2015.12.014. PMID 26944460.
- ↑ Apgar BS, Greenberg G (October 2000). "Using progestins in clinical practice". Am Fam Physician. 62 (8): 1839–46, 1849–50. PMID 11057840.
- 1 2 Goletiani NV, Keith DR, Gorsky SJ (2007). "Progesterone: review of safety for clinical studies". Exp Clin Psychopharmacol. 15 (5): 427–44. doi:10.1037/1064-1297.15.5.427. PMID 17924777.
- ↑ Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Strömberg J, Timby E, van Broekhoven F, van Wingen G (2014). "Allopregnanolone and mood disorders". Prog. Neurobiol. 113: 88–94. doi:10.1016/j.pneurobio.2013.07.005. PMID 23978486. S2CID 207407084.
- 1 2 3 4 5 6 7 8 Moore E, Wisniewski A, Dobs A (August 2003). "Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects". The Journal of Clinical Endocrinology and Metabolism. 88 (8): 3467–73. doi:10.1210/jc.2002-021967. PMID 12915619.
- 1 2 3 Davey DA (March 2018). "Menopausal hormone therapy: a better and safer future". Climacteric. 21 (5): 454–461. doi:10.1080/13697137.2018.1439915. PMID 29526116. S2CID 3850275.
- ↑ Raj R, Korja M, Koroknay-Pál P, Niemelä M (2018). "Multiple meningiomas in two male-to-female transsexual patients with hormone replacement therapy: A report of two cases and a brief literature review". Surg Neurol Int. 9: 109. doi:10.4103/sni.sni_22_18. PMC 5991277. PMID 29930875.
- ↑ Nota NM, Wiepjes CM, de Blok CJ, Gooren LJ, Peerdeman SM, Kreukels BP, den Heijer M (July 2018). "The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment". Brain. 141 (7): 2047–2054. doi:10.1093/brain/awy108. PMID 29688280. S2CID 19934721.
- ↑ Kuhl H (2011). "Pharmacology of Progestogens" (PDF). Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology. 8 (1): 157–177.
- ↑ Kuhl H, Schneider HP (August 2013). "Progesterone--promoter or inhibitor of breast cancer". Climacteric. 16 Suppl 1: 54–68. doi:10.3109/13697137.2013.768806. PMID 23336704. S2CID 20808536.
- 1 2 de Ziegler D, Fanchin R (2000). "Progesterone and progestins: applications in gynecology". Steroids. 65 (10–11): 671–9. doi:10.1016/S0039-128X(00)00123-9. PMID 11108875. S2CID 5867301.
- 1 2 Hermann AC, Nafziger AN, Victory J, Kulawy R, Rocci ML, Bertino JS (2005). "Over-the-counter progesterone cream produces significant drug exposure compared to a food and drug administration-approved oral progesterone product". J Clin Pharmacol. 45 (6): 614–9. doi:10.1177/0091270005276621. PMID 15901742. S2CID 28399314.
- ↑ Tollan A, Oian P, Kjeldsen SE, Eide I, Maltau JM (1993). "Progesterone reduces sympathetic tone without changing blood pressure or fluid balance in men". Gynecol. Obstet. Invest. 36 (4): 234–8. doi:10.1159/000292636. PMID 8300009.
- ↑ Unfer, Vittorio; di Renzo, Gian; Gerli, Sandro; Casini, Maria (2006). "The Use of Progesterone in Clinical Practice: Evaluation of its Efficacy in Diverse Indications Using Different Routes of Administration". Current Drug Therapy. 1 (2): 211–219. doi:10.2174/157488506776930923. ISSN 1574-8855.
- ↑ Brady BM, Anderson RA, Kinniburgh D, Baird DT (2003). "Demonstration of progesterone receptor-mediated gonadotrophin suppression in the human male". Clin. Endocrinol. (Oxf). 58 (4): 506–12. doi:10.1046/j.1365-2265.2003.01751.x. PMID 12641635. S2CID 12567639.
- ↑ A. Wayne Meikle (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 383, 389. ISBN 978-1-59259-700-0.
- ↑ Paynter MJ (March 2019). "Medication and Facilitation of Transgender Women's Lactation". J Hum Lact. 35 (2): 239–243. doi:10.1177/0890334419829729. PMID 30840524. S2CID 73466659.
- ↑ Telis, Leon; Baum, Stephanie; Singer, Tomer; Berookhim, Boback M. (2019). "Fertility Issues in Transgender Care". Transgender Medicine. Contemporary Endocrinology. pp. 197–212. doi:10.1007/978-3-030-05683-4_11. ISBN 978-3-030-05682-7. ISSN 2523-3785. S2CID 151135327.
- 1 2 Kozlov GI, Mel'nichenko GA, Golubeva IV (1985). "Sluchai laktorei u bol'nogo muzhskogo pola s transseksualizmom" [Case of galactorrhea in a transsexual male patient]. Probl Endokrinol (Mosk) (in Russian). 31 (1): 37–8. ISSN 0375-9660. PMID 4039061.
[...] castration and feminizing plastic surgery of the external genitalia was performed [...] Some time after the operation, the patient developed a renewed interest in life. After the surgical and hormonal correction, the patient irresistibly developed maternal instincts. Unmarried, the patient obtained permission for the adoption of a child, simulated pregnancy, and was discharged from the maternity hospital with a son. From the first days after the “birth”, galactorrhea sharply increased, and spontaneous outflow of milk appeared, with galactorrhea (+++). The baby was breastfed up to 6 months of age. [...] Our message is the second in the world literature describing galactorrhea in a male patient with transsexualism. The first description of this kind was made in 1983 by R. [Flückiger] et al. (6). This observation demonstrates the independence of the mechanism of lactation development from one’s genetic sex and is alarming with regard to the possibility of drug-induced galactorrhea development in men.
- ↑ Foss, GL (January 1956). "Abnormalities of form and function of the human breast". Journal of Endocrinology. 14 (1): R6–R9.
Based on the theories of lactogenesis and stimulated by the success of Lyons, Li, Johnson & Cole [1955], who succeeded in producing lactation in male rats, an attempt was made to initiate lactogenesis in a male transvestist. Six years ago this patient had been given oestrogens. Both testes and penis were then removed and an artificial vagina was constructed by plastic surgery. The patient was implanted with 500 mg oestradiol in September 1954, and 600 mg in July 1955. The breasts were then developed more intensively with daily injections of oestradiol dipropionate and progesterone for 6 weeks. Immediately following withdrawal of this treatment, prolactin 22·9 mg was injected daily for 3 days without effect. After a second month on oestradiol and progesterone daily, combined injections of prolactin and somatotrophin were given for 4 days and suction was applied by a breast pump-four times daily. On the 4th and 5th days a few drops of colostrum were expressed from the right nipple.
- ↑ Harold Gardiner-Hill (1958). Modern Trends in Endocrinology. Butterworth. p. 192.
Recently, an attempt has been made by Foss (1956) to initiate lactation in a castrated male transvestist. He was given an implant of 500 milligrams of oestradiol, and 10 months later, a further 600 milligrams of oestradiol, followed by daily injections of oestradiol dipropionate and progesterone for 6 weeks. Immediately after withdrawal of this treatment, 22·9 milligrams of prolactin were injected daily for 3 days but without effect. After a second month of treatment with oestradiol and progesterone daily, he was given combined injections of prolactin and somatotrophin for 4 days, suction with a breast-pump being employed 4 times daily. On the fourth and fifth days a few drops of colostrum were expressed from the right nipple. There is a possible application here of modern hormone knowledge to man, and further trials would be of interest.
- ↑ Edward Flückiger; Emilio Del Pozo; Klaus von Werder (1982). Prolactin: Physiology, Pharmacology, and Clinical Findings. Springer-Verlag. p. 13. ISBN 978-3-540-11071-2.
[...] An observation (Wyss and Del Pozo unpublished) in a male transsexual showed that induction of lactation can be similarly achieved in the human male. [...]
- ↑ Carla A. Pfeffer (2017). Queering Families: The Postmodern Partnerships of Cisgender Women and Transgender Men. Oxford University Press. pp. 19–. ISBN 978-0-19-990805-9.
Just 2 years later, Winfrey would feature another interview that elicited many of the same audience reactions. In this 2010 episode, lesbian partners Dr. Christine McGinn and Lisa Bortz beamed with joy as they held their infant twins. Again, audience members' jaws dropped when it was revealed that beautiful Christine was a male-to-female transsexual who used to be a handsome military officer Chris, and that Lisa had given birth to the couple's biological children using sperm Chris banked prior to gender confirmation surgeries.10 And it was Winfrey's chin that nearly hit the floor as she watched video of Christine breastfeeding the couples' children (the episode is referred to online as "The Mom Who Fathered Her Own Children"). [...]
- ↑ Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW (September 2010). "Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life". J Sex Med. 7 (9): 2996–3010. doi:10.1111/j.1743-6109.2010.01902.x. PMID 20626600.
- ↑ Higano CS (February 2003). "Side effects of androgen deprivation therapy: monitoring and minimizing toxicity". Urology. 61 (2 Suppl 1): 32–8. doi:10.1016/S0090-4295(02)02397-X. PMID 12667885.
- ↑ Higano CS (October 2012). "Sexuality and intimacy after definitive treatment and subsequent androgen deprivation therapy for prostate cancer". J. Clin. Oncol. 30 (30): 3720–5. doi:10.1200/JCO.2012.41.8509. PMID 23008326.
- ↑ Eberhard Nieschlag; Hermann Behre (29 June 2013). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 54–. ISBN 978-3-662-04491-9.
- 1 2 Fisher, Alessandra Daphne; Maggi, Mario (2015). "Endocrine Treatment of Transsexual Male-to-Female Persons". Management of Gender Dysphoria. pp. 83–91. doi:10.1007/978-88-470-5696-1_10. ISBN 978-88-470-5695-4.
- 1 2 Radix, Asa E. (2016). "Medical Transition for Transgender Individuals". Lesbian, Gay, Bisexual, and Transgender Healthcare. pp. 351–361. doi:10.1007/978-3-319-19752-4_19. ISBN 978-3-319-19751-7.
- ↑ de, Blok Christel; Klaver, Maartje; Nota, Nienke; Dekker, Marieke; den, Heijer Martin (2016). "Breast development in male-to-female transgender patients after one year cross-sex hormonal treatment". Endocrine Abstracts. doi:10.1530/endoabs.41.GP146. ISSN 1479-6848.
- ↑ de Blok CJ, Klaver M, Wiepjes CM, Nota NM, Heijboer AC, Fisher AD, Schreiner T, T'Sjoen G, den Heijer M (February 2018). "Breast Development in Transwomen After 1 Year of Cross-Sex Hormone Therapy: Results of a Prospective Multicenter Study". J. Clin. Endocrinol. Metab. 103 (2): 532–538. doi:10.1210/jc.2017-01927. PMID 29165635. S2CID 3716975.
- 1 2 3 4 5 6 Asscheman H, Gooren LJ (1992). "Hormone Treatment in Transsexuals". Archived from the original on 3 June 2012. Retrieved 13 June 2008.
- ↑ Meikle, James. "Breast regrowth procedure trialled for mastectomy patients". The Guardian. Retrieved 17 January 2015.
- 1 2 3 4 5 van Kesteren, Paul J. M. (16 April 2002). Recent Advanced in Gender Dysphoria, Gender Identity Disorder: Towards a Uniform Treatment Approach. Conference of the Royal Society of Medicine, Sexual Health and Reproductive Medicine Section. London, United Kingdom.
- 1 2 3 Kirk, Sheila (1999). Feminizing Hormonal Therapy For The Transgendered. Pittsburgh, PA: Together Lifeworks. p. 38. ISBN 1887796045.
- 1 2 3 Giltay EJ, Gooren LJ (August 2000). "Effects of sex steroid deprivation/administration on hair growth and skin sebum production in transsexual males and females". Journal of Clinical Endocrinology and Metabolism. 85 (8): 2913–21. doi:10.1210/jc.85.8.2913. PMID 10946903.
- ↑ Randall VA, Hibberts NA, Thornton MJ, Hamada K, Merrick AE, Kato S, Jenner TJ, De Oliveira I, Messenger AG (2000). "The hair follicle: a paradoxical androgen target organ". Horm. Res. 54 (5–6): 243–50. doi:10.1159/000053266. PMID 11595812. S2CID 42826314.
- ↑ Leach NE, Wallis NE, Lothringer LL, Olson JA (May 1971). "Corneal hydration changes during the normal menstrual cycle--a preliminary study". The Journal of Reproductive Medicine. 6 (5): 201–4. PMID 5094729.
- ↑ Kiely PM, Carney LG, Smith G (October 1983). "Menstrual cycle variations of corneal topography and thickness" (PDF). American Journal of Optometry and Physiological Optics. 60 (10): 822–9. doi:10.1097/00006324-198310000-00003. PMID 6650653. S2CID 43222063.
- ↑ Gurwood AS, Gurwood I, Gubman DT, Brzezicki LJ (January 1995). "Idiosyncratic ocular symptoms associated with the estradiol transdermal estrogen replacement patch system". Optometry and Vision Science. 72 (1): 29–33. doi:10.1097/00006324-199501000-00006. PMID 7731653.
- ↑ Krenzer KL, Dana MR, Ullman MD, et al. (December 2000). "Effect of androgen deficiency on the human meibomian gland and ocular surface". The Journal of Clinical Endocrinology and Metabolism. 85 (12): 4874–82. doi:10.1210/jcem.85.12.7072. PMID 11134156.
- ↑ Sullivan DA, Sullivan BD, Evans JE, et al. (June 2002). "Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye". Annals of the New York Academy of Sciences. 966 (1): 211–22. Bibcode:2002NYASA.966..211S. doi:10.1111/j.1749-6632.2002.tb04217.x. PMID 12114274. S2CID 22281698.
- ↑ Sullivan BD, Evans JE (December 2002). "Complete androgen insensitivity syndrome: effect on human meibomian gland secretions". Archives of Ophthalmology. 120 (12): 1689–1699. doi:10.1001/archopht.120.12.1689. PMID 12470144.
- ↑ Cermak JM, Krenzer KL, Sullivan RM, Dana MR, Sullivan DA (August 2003). "Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface?". Cornea. 22 (6): 516–21. doi:10.1097/00003226-200308000-00006. PMID 12883343. S2CID 29374194.
- ↑ Oprea L, Tiberghien A, Creuzot-Garcher C, Baudouin C (October 2004). "Influence des hormones sur le film lacrymal" [Hormonal regulatory influence in tear film]. Journal Français d'Ophtalmologie (in French). 27 (8): 933–41. doi:10.1016/S0181-5512(04)96241-9. PMID 15547478.
- ↑ Peterson's Principles of Oral and Maxillofacial Surgery. PMPH-USA. 2012. pp. 1209–. ISBN 978-1-60795-111-7.
- 1 2 Nguyen, Hillary B.; Chavez, Alexis M.; Lipner, Emily; Hantsoo, Liisa; Kornfield, Sara L.; Davies, Robert D.; Epperson, C. Neill (2018). "Gender-Affirming Hormone Use in Transgender Individuals: Impact on Behavioral Health and Cognition". Current Psychiatry Reports. 20 (12): 110. doi:10.1007/s11920-018-0973-0. ISSN 1523-3812. PMC 6354936. PMID 30306351.
- ↑ Garcia, Maurice; Zaliznyak, Michael (2020). "Mp45-20 Effects of Feminizing Hormone Therapy on Sexual Function of Transgender Women". The Journal of Urology. 203 (Supplement 4): e672. doi:10.1097/JU.0000000000000900.020. S2CID 218946871.
- 1 2 3 4 Klein C., Gorzalka B.B. (2009). "Sexual functioning in transsexuals following hormone therapy and genital surgery: A review". Journal of Sexual Medicine. 6 (11): 2922–2939. doi:10.1111/j.1743-6109.2009.01370.x. PMID 20092545.
- 1 2 Smith, Elke Stefanie; Junger, Jessica; Derntl, Birgit; Habel, Ute (2015). "The transsexual brain – A review of findings on the neural basis of transsexualism". Neuroscience & Biobehavioral Reviews. 59: 251–266. doi:10.1016/j.neubiorev.2015.09.008. ISSN 0149-7634. PMID 26429593. S2CID 23913935.
- ↑ Guillamon, Antonio; Junque, Carme; Gómez-Gil, Esther (2016). "A Review of the Status of Brain Structure Research in Transsexualism". Archives of Sexual Behavior. 45 (7): 1615–1648. doi:10.1007/s10508-016-0768-5. ISSN 0004-0002. PMC 4987404. PMID 27255307.
- ↑ Mueller, Sven C.; De Cuypere, Griet; T’Sjoen, Guy (2017). "Transgender Research in the 21st Century: A Selective Critical Review From a Neurocognitive Perspective". American Journal of Psychiatry. 174 (12): 1155–1162. doi:10.1176/appi.ajp.2017.17060626. hdl:1854/LU-8542009. ISSN 0002-953X. PMID 29050504.
- ↑ Nguyen HB, Loughead J, Lipner E, Hantsoo L, Kornfield SL, Epperson CN (January 2019). "What has sex got to do with it? The role of hormones in the transgender brain". Neuropsychopharmacology. 44 (1): 22–37. doi:10.1038/s41386-018-0140-7. PMC 6235900. PMID 30082887.
- ↑ Kilpatrick, Lisa A.; Holmberg, Mats; Manzouri, Amirhosein; Savic, Ivanka (2019). "Cross sex hormone treatment is linked with a reversal of cerebral patterns associated with gender dysphoria to the baseline of cisgender controls". European Journal of Neuroscience. 50 (8): 3269–3281. doi:10.1111/ejn.14420. ISSN 0953-816X. PMC 7329231. PMID 30991464.
- ↑ Henriksson P, Eriksson A, Stege R, Collste L, Pousette A, von Schoultz B, Carlström K (1988). "Cardiovascular follow-up of patients with prostatic cancer treated with single-drug polyestradiol phosphate". Prostate. 13 (3): 257–61. doi:10.1002/pros.2990130308. PMID 3211807. S2CID 20686808.
- ↑ von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function--metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738. S2CID 21510744.
- ↑ Asscheman H, Gooren LJ, Eklund PL (September 1989). "Mortality and morbidity in transsexual patients with cross-gender hormone treatment". Metab. Clin. Exp. 38 (9): 869–73. doi:10.1016/0026-0495(89)90233-3. PMID 2528051.
- ↑ Aro J, Haapiainen R, Rasi V, Rannikko S, Alfthan O (1990). "The effect of parenteral estrogen versus orchiectomy on blood coagulation and fibrinolysis in prostatic cancer patients". Eur. Urol. 17 (2): 161–5. doi:10.1159/000464026. PMID 2178941.
- ↑ Henriksson P, Blombäck M, Eriksson A, Stege R, Carlström K (March 1990). "Effect of parenteral oestrogen on the coagulation system in patients with prostatic carcinoma". Br J Urol. 65 (3): 282–5. doi:10.1111/j.1464-410X.1990.tb14728.x. PMID 2110842.
- ↑ Aro J (1991). "Cardiovascular and all-cause mortality in prostatic cancer patients treated with estrogens or orchiectomy as compared to the standard population". Prostate. 18 (2): 131–7. doi:10.1002/pros.2990180205. PMID 2006119. S2CID 27915767.
- ↑ Henriksson P, Stege R (1991). "Cost comparison of parenteral estrogen and conventional hormonal treatment in patients with prostatic cancer". Int J Technol Assess Health Care. 7 (2): 220–5. doi:10.1017/S0266462300005110. PMID 1907600.
- ↑ Henriksson P (1991). "Estrogen in patients with prostatic cancer. An assessment of the risks and benefits". Drug Saf. 6 (1): 47–53. doi:10.2165/00002018-199106010-00005. PMID 2029353. S2CID 39861824.
- ↑ Caine YG, Bauer KA, Barzegar S, ten Cate H, Sacks FM, Walsh BW, Schiff I, Rosenberg RD (October 1992). "Coagulation activation following estrogen administration to postmenopausal women". Thromb. Haemost. 68 (4): 392–5. doi:10.1055/s-0038-1646283. PMID 1333098.
- ↑ Stege R, Sander S (March 1993). "Endokrin behandling av prostatacancer. En renessanse for parenteralt østrogen" [Endocrine treatment of prostatic cancer. A renaissance for parenteral estrogen]. Tidsskr. Nor. Laegeforen. (in Norwegian). 113 (7): 833–5. PMID 8480286.
- ↑ Stege R, Carlström K, Hedlund PO, Pousette A, von Schoultz B, Henriksson P (September 1995). "Intramuskuläres Depotöstrogen (Estradurin) in der Behandlung von Patienten mit Prostatakarzinom. Historische Aspekte, Wirkungsmechanismus, Resultate und aktueller klinischer Stand" [Intramuscular depot estrogens (Estradurin) in treatment of patients with prostate carcinoma. Historical aspects, mechanism of action, results and current clinical status]. Urologe A (in German). 34 (5): 398–403. ISSN 0340-2592. PMID 7483157.
- ↑ Henriksson P, Carlström K, Pousette A, Gunnarsson PO, Johansson CJ, Eriksson B, Altersgård-Brorsson AK, Nordle O, Stege R (July 1999). "Time for revival of estrogens in the treatment of advanced prostatic carcinoma? Pharmacokinetics, and endocrine and clinical effects, of a parenteral estrogen regimen". Prostate. 40 (2): 76–82. doi:10.1002/(SICI)1097-0045(19990701)40:2<76::AID-PROS2>3.0.CO;2-Q. PMID 10386467.
- ↑ Hedlund PO, Henriksson P (March 2000). "Parenteral estrogen versus total androgen ablation in the treatment of advanced prostate carcinoma: effects on overall survival and cardiovascular mortality. The Scandinavian Prostatic Cancer Group (SPCG)-5 Trial Study". Urology. 55 (3): 328–33. doi:10.1016/S0090-4295(99)00580-4. PMID 10699602.
- ↑ Hedlund PO, Ala-Opas M, Brekkan E, Damber JE, Damber L, Hagerman I, Haukaas S, Henriksson P, Iversen P, Pousette A, Rasmussen F, Salo J, Vaage S, Varenhorst E (2002). "Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer -- Scandinavian Prostatic Cancer Group (SPCG) Study No. 5". Scand. J. Urol. Nephrol. 36 (6): 405–13. doi:10.1080/003655902762467549. PMID 12623503. S2CID 2799580.
- ↑ Scarabin PY, Oger E, Plu-Bureau G (August 2003). "Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk". Lancet. 362 (9382): 428–32. doi:10.1016/S0140-6736(03)14066-4. PMID 12927428. S2CID 45789951.
- ↑ Straczek C, Oger E, Yon de Jonage-Canonico MB, Plu-Bureau G, Conard J, Meyer G, Alhenc-Gelas M, Lévesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY (November 2005). "Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration". Circulation. 112 (22): 3495–500. doi:10.1161/CIRCULATIONAHA.105.565556. PMID 16301339. S2CID 13587974.
- ↑ Basurto L, Saucedo R, Zárate A, Martínez C, Gaminio E, Reyes E, Hernandez M (2006). "Effect of pulsed estrogen therapy on hemostatic markers in comparison with oral estrogen regimen in postmenopausal women". Gynecol. Obstet. Invest. 61 (2): 61–4. doi:10.1159/000088603. PMID 16192735. S2CID 38375159.
- ↑ Hemelaar M, Rosing J, Kenemans P, Thomassen MC, Braat DD, van der Mooren MJ (July 2006). "Less effect of intranasal than oral hormone therapy on factors associated with venous thrombosis risk in healthy postmenopausal women". Arterioscler. Thromb. Vasc. Biol. 26 (7): 1660–6. doi:10.1161/01.ATV.0000224325.96659.53. PMID 16645152. S2CID 12778600.
- ↑ Hedlund PO, Damber JE, Hagerman I, Haukaas S, Henriksson P, Iversen P, Johansson R, Klarskov P, Lundbeck F, Rasmussen F, Varenhorst E, Viitanen J (2008). "Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer: part 2. Final evaluation of the Scandinavian Prostatic Cancer Group (SPCG) Study No. 5". Scand. J. Urol. Nephrol. 42 (3): 220–9. doi:10.1080/00365590801943274. PMID 18432528. S2CID 38638336.
- ↑ Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY (May 2008). "Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis". BMJ. 336 (7655): 1227–31. doi:10.1136/bmj.39555.441944.BE. PMC 2405857. PMID 18495631.
- ↑ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 753–. ISBN 978-1-4511-4847-3.
- ↑ Rosendale N, Goldman S, Ortiz GM, Haber LA (November 2018). "Acute Clinical Care for Transgender Patients: A Review". JAMA Intern Med. 178 (11): 1535–1543. doi:10.1001/jamainternmed.2018.4179. PMID 30178031. S2CID 52146607.
- ↑ Speed V, Roberts LN, Patel JP, Arya R (November 2018). "Venous thromboembolism and women's health". Br. J. Haematol. 183 (3): 346–363. doi:10.1111/bjh.15608. PMID 30334572. S2CID 52985304.
- 1 2 3 4 5 Khan J, Schmidt RL, Spittal MJ, Goldstein Z, Smock KJ, Greene DN (January 2019). "Venous Thrombotic Risk in Transgender Women Undergoing Estrogen Therapy: A Systematic Review and Metaanalysis". Clin. Chem. 65 (1): 57–66. doi:10.1373/clinchem.2018.288316. PMID 30602475.
- ↑ Heit JA (August 2015). "Epidemiology of venous thromboembolism". Nat Rev Cardiol. 12 (8): 464–74. doi:10.1038/nrcardio.2015.83. PMC 4624298. PMID 26076949.
- 1 2 Houlberg, Magda (2019). "Endocrinology, Hormone Replacement Therapy (HRT), and Aging". Transgender and Gender Nonconforming Health and Aging. pp. 21–35. doi:10.1007/978-3-319-95031-0_2. ISBN 978-3-319-95030-3. S2CID 81674566.
- 1 2 3 4 Arnold JD, Sarkodie EP, Coleman ME, Goldstein DA (November 2016). "Incidence of Venous Thromboembolism in Transgender Women Receiving Oral Estradiol". J Sex Med. 13 (11): 1773–1777. doi:10.1016/j.jsxm.2016.09.001. PMID 27671969.
- 1 2 Streed CG, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M (August 2017). "Cardiovascular Disease Among Transgender Adults Receiving Hormone Therapy: A Narrative Review". Ann. Intern. Med. 167 (4): 256–267. doi:10.7326/M17-0577. PMID 28738421. S2CID 207538881.
- 1 2 3 Eismann J, Heng YJ, Fleischmann-Rose K, Tobias AM, Phillips J, Wulf GM, Kansal KJ (February 2019). "Interdisciplinary Management of Transgender Individuals at Risk for Breast Cancer: Case Reports and Review of the Literature". Clin. Breast Cancer. 19 (1): e12–e19. doi:10.1016/j.clbc.2018.11.007. PMC 7083129. PMID 30527351.
- 1 2 Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ (December 2013). "Breast cancer development in transsexual subjects receiving cross-sex hormone treatment". J Sex Med. 10 (12): 3129–34. doi:10.1111/jsm.12319. PMID 24010586.
- 1 2 Brown GR, Jones KT (January 2015). "Incidence of breast cancer in a cohort of 5,135 transgender veterans". Breast Cancer Res. Treat. 149 (1): 191–8. doi:10.1007/s10549-014-3213-2. PMID 25428790. S2CID 10935304.
- 1 2 de Blok, Christel J M; Wiepjes, Chantal M; Nota, Nienke M; van Engelen, Klaartje; Adank, Muriel A; Dreijerink, Koen M A; Barbé, Ellis; Konings, Inge R H M; den Heijer, Martin (2019). "Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands". BMJ. 365: l1652. doi:10.1136/bmj.l1652. ISSN 0959-8138. PMC 6515308. PMID 31088823.
- ↑ Iwamoto, Sean J.; Defreyne, Justine; Rothman, Micol S.; Van Schuylenbergh, Judith; Van de Bruaene, Laurens; Motmans, Joz; T’Sjoen, Guy (2019). "Health considerations for transgender women and remaining unknowns: a narrative review". Therapeutic Advances in Endocrinology and Metabolism. 10: 204201881987116. doi:10.1177/2042018819871166. ISSN 2042-0188. PMC 6719479. PMID 31516689.
- ↑ Hartley RL, Stone JP, Temple-Oberle C (October 2018). "Breast cancer in transgender patients: A systematic review. Part 1: Male to female". Eur J Surg Oncol. 44 (10): 1455–1462. doi:10.1016/j.ejso.2018.06.035. PMID 30087072. S2CID 51936024.
- 1 2 3 Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B (2014). "Gynecomastia: Clinical evaluation and management". Indian J Endocrinol Metab. 18 (2): 150–8. doi:10.4103/2230-8210.129104. PMC 3987263. PMID 24741509.
- 1 2 Niewoehner CB, Schorer AE (2008). "Gynaecomastia and breast cancer in men". BMJ. 336 (7646): 709–13. doi:10.1136/bmj.39511.493391.BE. PMC 2276281. PMID 18369226.
- ↑ Christopher Li (11 November 2009). Breast Cancer Epidemiology. Springer Science & Business Media. pp. 266–. ISBN 978-1-4419-0685-4.
- ↑ Stella Pelengaris; Michael Khan (13 March 2013). The Molecular Biology of Cancer: A Bridge from Bench to Bedside. John Wiley & Sons. pp. 586–. ISBN 978-1-118-43085-9.
- ↑ Gilda Cardenosa (2004). Breast Imaging. Lippincott Williams & Wilkins. pp. 1–. ISBN 978-0-7817-4685-4.
- ↑ Jerome F. Strauss, III; Robert L. Barbieri (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 236–. ISBN 978-1-4557-2758-2.
- ↑ Hughes IA, Werner R, Bunch T, Hiort O (2012). "Androgen insensitivity syndrome". Semin. Reprod. Med. 30 (5): 432–42. doi:10.1055/s-0032-1324728. PMID 23044881.
- ↑ Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA (2008). "Cancer incidence in women with Turner syndrome in Great Britain: a national cohort study". Lancet Oncol. 9 (3): 239–46. doi:10.1016/S1470-2045(08)70033-0. PMID 18282803.
- 1 2 3 Gooren L, Morgentaler A (2014). "Prostate cancer incidence in orchidectomised male-to-female transsexual persons treated with oestrogens". Andrologia. 46 (10): 1156–60. doi:10.1111/and.12208. PMID 24329588. S2CID 1445627.
- 1 2 3 Turo R, Jallad S, Prescott S, Cross WR (2013). "Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy". Can Urol Assoc J. 7 (7–8): E544–6. doi:10.5489/cuaj.175. PMC 3758950. PMID 24032068.
- 1 2 McFarlane T, Zajac JD, Cheung AS (December 2018). "Gender-affirming hormone therapy and the risk of sex hormone-dependent tumours in transgender individuals-A systematic review". Clin. Endocrinol. (Oxf). 89 (6): 700–711. doi:10.1111/cen.13835. PMID 30107028. S2CID 52003943.
- 1 2 3 4 "Archived copy" (PDF). callen-lorde.org. Archived from the original (PDF) on 5 September 2019. Retrieved 15 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ McFarlane, Thomas; Zajac, Jeffrey D.; Cheung, Ada S. (2018). "Gender-affirming hormone therapy and the risk of sex hormone-dependent tumours in transgender individuals-A systematic review". Clinical Endocrinology. 89 (6): 700–711. doi:10.1111/cen.13835. ISSN 0300-0664. PMID 30107028. S2CID 52003943.
- ↑ Nota, Nienke M; Wiepjes, Chantal M; de Blok, Christel J M; Gooren, Louis J G; Peerdeman, Saskia M; Kreukels, Baudewijntje P C; den Heijer, Martin (2018). "The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment". Brain. 141 (7): 2047–2054. doi:10.1093/brain/awy108. ISSN 0006-8950. PMID 29688280. S2CID 19934721.
- 1 2 Mahfouda, Simone; Moore, Julia K; Siafarikas, Aris; Hewitt, Timothy; Ganti, Uma; Lin, Ashleigh; Zepf, Florian Daniel (2019). "Gender-affirming hormones and surgery in transgender children and adolescents". The Lancet Diabetes & Endocrinology. 7 (6): 484–498. doi:10.1016/S2213-8587(18)30305-X. ISSN 2213-8587. PMID 30528161. S2CID 54478571.
- ↑ Bisson, Jason R.; Chan, Kelly J.; Safer, Joshua D. (2018). "Prolactin levels do not rise among transgender women treated with estradiol and spironolactone". Endocrine Practice. 24 (7): 646–651. doi:10.4158/EP-2018-0101. ISSN 1530-891X. PMID 29708436. S2CID 14022275.
- ↑ Elizabeth Siegel Watkins (16 April 2007). The Estrogen Elixir: A History of Hormone Replacement Therapy in America. JHU Press. pp. 10–. ISBN 978-0-8018-8602-7.
- 1 2 Hamburger C, Sturup GK, Dahl-Iversen E (May 1953). "Transvestism; hormonal, psychiatric, and surgical treatment". J Am Med Assoc. 152 (5): 391–6. doi:10.1001/jama.1953.03690050015006. PMID 13044539.
- 1 2 3 Institute of Medicine; Board on the Health of Select Populations; Committee on Lesbian, Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities (24 June 2011). The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. National Academies Press. pp. 70–. ISBN 978-0-309-21065-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Bullough VL (September 1975). "Transsexualism in history". Arch Sex Behav. 4 (5): 561–71. doi:10.1007/bf01542134. PMID 1103789. S2CID 36577490.
- ↑ Dallas Denny (13 May 2013). Current Concepts in Transgender Identity. Routledge. pp. 15–. ISBN 978-1-134-82110-5.
- ↑ Susan Stryker; Associate Professor of Gender and Women's Studies Susan Stryker; Stephen Whittle (2006). The Transgender Studies Reader. Taylor & Francis. pp. 363–. ISBN 978-0-415-94709-1.
- 1 2 3 Gooren, Louis; Asscheman, Henk (2014). "Sex Reassignment: Endocrinological Interventions in Adults with Gender Dysphoria". Gender Dysphoria and Disorders of Sex Development. Focus on Sexuality Research. pp. 277–297. doi:10.1007/978-1-4614-7441-8_14. ISBN 978-1-4614-7440-1. ISSN 2195-2264.
- ↑ Baudewijntje P.C. Kreukels; Thomas D. Steensma; Annelou L.C. de Vries (1 July 2013). Gender Dysphoria and Disorders of Sex Development: Progress in Care and Knowledge. Springer Science & Business Media. pp. 279–. ISBN 978-1-4614-7441-8.
- ↑ Benjamin H (July 1964). "Clinical aspects of transsexualism in the male and female". Am J Psychother. 18 (3): 458–69. doi:10.1176/appi.psychotherapy.1964.18.3.458. PMID 14173773.
- 1 2 3 Harry Benjamin; Gobind Behari Lal; Richard Green; Robert E. L. Masters (1966). The Transsexual Phenomenon. Ace Publishing Company.
- 1 2 3 Benjamin, Harry (1967). "Transvestism and Transsexualism in the male and female1". Journal of Sex Research. 3 (2): 107–127. doi:10.1080/00224496709550519. ISSN 0022-4499.
- 1 2 3 Hamburger, Christian; Benjamin, Harry (1969). "Endocrine Treatment of Male and Female Transsexualism / Appendix for the Practicing Physician: Suggestions and Guidelines for the Management of Transsexuals". In Money, John; Green, Richard (eds.). Transsexualism and Sex Reassignment. Johns Hopkins Press. pp. 291–307. ISBN 9780801810381. OCLC 6866559.
- ↑ Schaefer LC, Wheeler CC (February 1995). "Harry Benjamin's first ten cases (1938-1953): a clinical historical note". Arch Sex Behav. 24 (1): 73–93. doi:10.1007/bf01541990. PMID 7733806. S2CID 31571764.
- ↑ Abbie E. Goldberg (13 April 2016). The SAGE Encyclopedia of LGBTQ Studies. SAGE Publications. pp. 1211–. ISBN 978-1-4833-7132-0.
- ↑ Susan Stryker; Stephen Whittle (18 October 2013). The Transgender Studies Reader. Routledge. pp. 45–. ISBN 978-1-135-39884-2.
- ↑ Edgerton MT, Knorr NJ, Callison JR (January 1970). "The surgical treatment of transsexual patients. Limitations and indications". Plast. Reconstr. Surg. 45 (1): 38–46. doi:10.1097/00006534-197001000-00006. PMID 4902840. S2CID 27318408.
- ↑ Ekins, Richard (2016). "Science, Politics and Clinical Intervention: Harry Benjamin, Transsexualism and the Problem of Heteronormativity". Sexualities. 8 (3): 306–328. doi:10.1177/1363460705049578. ISSN 1363-4607. S2CID 143544267.
- 1 2 3 4 Meyer, Walter J.; Walker, Paul A.; Suplee, Zelda R. (1981). "A survey of transsexual hormonal treatment in twenty gender‐treatment centers". The Journal of Sex Research. 17 (4): 344–349. doi:10.1080/00224498109551125. ISSN 0022-4499.
- ↑ "International Symposia - WPATH World Professional Association for Transgender Health".
- ↑ Green, R., & Money, J. (1969). Transsexualism and Sex Reassignment. Johns Hopkins University Press. https://scholar.google.com/scholar?cluster=8048451400842332421 https://books.google.com/books?id=pdBrAAAAMAAJ
- ↑ Benjamin, H., & Ihlenfeld, C. L. (1970). The Nature and Treatment of Transsexualism. Medical Opinion and Review, 6(11), 24–35. "Fortunately, the first medical textbook in this field, Transsexualism and Sex Reassignment, edited by Richard Green and John Money (Johns Hopkins Press, Baltimore, 1969), is now available." https://scholar.google.com/scholar?cluster=4496539582926153095
- ↑ Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP, Tangpricha V, Montori VM (September 2009). "Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline". J. Clin. Endocrinol. Metab. 94 (9): 3132–54. doi:10.1210/jc.2009-0345. PMID 19509099.
- 1 2 3 4 Prior JC, Vigna YM, Watson D (February 1989). "Spironolactone with physiological female steroids for presurgical therapy of male-to-female transsexualism". Arch Sex Behav. 18 (1): 49–57. doi:10.1007/BF01579291. PMID 2540730. S2CID 22802329.
- 1 2 3 Prior JC, Vigna YM, Watson D, Diewold P, Robinow O. "Spironolactone in the presurgical therapy of male to female transsexuals: Philosophy and experience of the Vancouver Gender Dysphoria Clinic". Journal of Sex Information & Education Council of Canada (1): 1–7.
- ↑ Moore, Eva; Wisniewski, Amy; Dobs, Adrian (2003). "Endocrine Treatment of Transsexual People: A Review of Treatment Regimens, Outcomes, and Adverse Effects". The Journal of Clinical Endocrinology & Metabolism. 88 (8): 3467–3473. doi:10.1210/jc.2002-021967. ISSN 0021-972X. PMID 12915619.
- ↑ Steinbeck, A. W. (1977). "Of Homosexuality: The Current State of Knowledge". Journal of Christian Education. os-20 (2): 58–82. doi:10.1177/002196577702000204. ISSN 0021-9657. S2CID 149168765.
- ↑ Zingg, E.; König, M.; Cornu, F.; Wildholz, A.; Blaser, A. (1980). "Transsexualismus: Erfahrungen mit der operativen Korrektur bei männlichen Transsexuellen" [Transsexualism: Experience with surgical correction in male transsexuals]. Aktuelle Urologie. 11 (2): 67–77. doi:10.1055/s-2008-1062961. ISSN 0001-7868.
- 1 2 Jequier AM, Bullimore NJ, Bishop MJ (1989). "Cyproterone acetate and a small dose of oestrogen in the pre-operative management of male transsexuals. A report of three cases". Andrologia. 21 (5): 456–61. doi:10.1111/j.1439-0272.1989.tb02447.x. PMID 2530920. S2CID 30370123.
- ↑ Kuiper AJ, Cohen-Kettenis PT, Van der Reyt F (1985). "Transsexuality in The Netherlands. Some medical and legal aspects". Med Law. 4 (4): 373–8. PMID 3900616.
- ↑ Dahl, Marshall; Feldman, Jamie L.; Goldberg, Joshua M.; Jaberi, Afshin (2006). "Physical Aspects of Transgender Endocrine Therapy". International Journal of Transgenderism. 9 (3–4): 111–134. doi:10.1300/J485v09n03_06. ISSN 1553-2739. S2CID 146232471.
- ↑ Gooren LJ, van der Veen EA, van Kessel H, Harmsen-Louman W, Wiegel AR (1984). "Androgens in the feedback regulation of gonadotropin secretion in men: effects of administration of dihydrotestosterone to eugonadal and agonadal subjects and of spironolactone to eugonadal subjects". Andrologia. 16 (4): 289–98. doi:10.1111/j.1439-0272.1984.tb00286.x. PMID 6433746. S2CID 32546312.
- ↑ Schaefer, L. C., Wheeler, C. C., & Futterweit, W. (1995). Gender identity disorders (transsexualism). In Rosenthal, N. E., & Gabbard, G. O. Treatment of Psychiatric Disorders, 2nd Edition, Volume 2 (pp. ). Washington, D.C.: American Psychiatric Press.
Further reading
- Gooren LJ, Giltay EJ, Bunck MC (January 2008). "Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience". J. Clin. Endocrinol. Metab. 93 (1): 19–25. doi:10.1210/jc.2007-1809. PMID 17986639.
- Gooren LJ (March 2011). "Clinical practice. Care of transsexual persons". N. Engl. J. Med. 364 (13): 1251–7. doi:10.1056/NEJMcp1008161. PMID 21449788.
- Wierckx K, Gooren L, T'Sjoen G (2014). "Clinical review: Breast development in trans women receiving cross-sex hormones". J Sex Med. 11 (5): 1240–7. doi:10.1111/jsm.12487. PMID 24618412.
- Fabris B, Bernardi S, Trombetta C (March 2015). "Cross-sex hormone therapy for gender dysphoria". J. Endocrinol. Invest. 38 (3): 269–82. doi:10.1007/s40618-014-0186-2. PMID 25403429. S2CID 207503049.
- Tangpricha V, den Heijer M (April 2017). "Oestrogen and anti-androgen therapy for transgender women". Lancet Diabetes Endocrinol. 5 (4): 291–300. doi:10.1016/S2213-8587(16)30319-9. PMC 5366074. PMID 27916515.
- Unger CA (December 2016). "Hormone therapy for transgender patients". Transl Androl Urol. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
- Wesp LM, Deutsch MB (March 2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". Psychiatr. Clin. North Am. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
External links
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG (November 2017). "Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline" (PDF). J. Clin. Endocrinol. Metab. 102 (11): 3869–3903. doi:10.1210/jc.2017-01658. PMID 28945902. S2CID 3726467.
- Coleman, E.; Bockting, W.; Botzer, M.; Cohen-Kettenis, P.; DeCuypere, G.; Feldman, J.; Fraser, L.; Green, J.; Knudson, G.; Meyer, W. J.; Monstrey, S.; Adler, R. K.; Brown, G. R.; Devor, A. H.; Ehrbar, R.; Ettner, R.; Eyler, E.; Garofalo, R.; Karasic, D. H.; Lev, A. I.; Mayer, G.; Meyer-Bahlburg, H.; Hall, B. P.; Pfaefflin, F.; Rachlin, K.; Robinson, B.; Schechter, L. S.; Tangpricha, V.; van Trotsenburg, M.; Vitale, A.; Winter, S.; Whittle, S.; Wylie, K. R.; Zucker, K. (2012). "Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7" (PDF). International Journal of Transgenderism. 13 (4): 165–232. doi:10.1080/15532739.2011.700873. ISSN 1553-2739. S2CID 39664779.
- Deutsch M (17 June 2016). "Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People" (2nd ed.). University of California, San Francisco: Center of Excellence for Transgender Health. p. 28.
{{cite web}}: CS1 maint: url-status (link) - Dahl, M; Feldman, JL; Goldberg, J; Jaberi, A (2015). "Endocrine Therapy for Transgender Adults in British Columbia: Suggested Guidelines" (PDF). Vancouver Coastal Health. Retrieved 15 August 2018.
- Bourns, Amy (2015). "Guidelines and Protocols for Comprehensive Primary Care for Trans Clients" (PDF). Sherbourne Health Centre. Retrieved 15 August 2018.
- Transgender HRT Research Repository