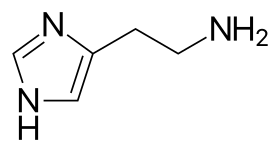
Histamine H3 receptor
Histamine H3 receptors are expressed in the central nervous system and to a lesser extent the peripheral nervous system, where they act as autoreceptors in presynaptic histaminergic neurons and control histamine turnover by feedback inhibition of histamine synthesis and release.[5] The H3 receptor has also been shown to presynaptically inhibit the release of a number of other neurotransmitters (i.e. it acts as an inhibitory heteroreceptor) including, but probably not limited to dopamine, GABA, acetylcholine, noradrenaline, histamine and serotonin.
The gene sequence for H3 receptors expresses only about 22% and 20% homology with both H1 and H2 receptors respectively.
There is much interest in the histamine H3 receptor as a potential therapeutic target because of its involvement in the neuronal mechanism behind many cognitive disorders and especially its location in the central nervous system.[6][7]
Tissue distribution
Function
Like all histamine receptors, the H3 receptor is a G-protein coupled receptor. The H3 receptor is coupled to the Gi G-protein, so it leads to inhibition of the formation of cAMP. Also, the β and γ subunits interact with N-type voltage gated calcium channels, to reduce action potential mediated influx of calcium and hence reduce neurotransmitter release. H3 receptors function as presynaptic autoreceptors on histamine-containing neurons.[8]
The diverse expression of H3 receptors throughout the cortex and subcortex indicates its ability to modulate the release of a large number of neurotransmitters.
H3 receptors are thought to play a part in the control of satiety.[9]
Isoforms
There are at least six H3 receptor isoforms in the human, and more than 20 discovered so far.[10] In rats there have been six H3receptor subtypes identified so far. Mice also have three reported isoforms.[11] These subtypes all have subtle difference in their pharmacology (and presumably distribution, based on studies in rats) but the exact physiological role of these isoforms is still unclear.
Pharmacology
Agonists
There are currently no therapeutic products acting as selective agonists for H3 receptors, although there are several compounds used as research tools which are reasonably selective agonists. Some examples are:
- (R)-α-methylhistamine
- Cipralisant (initially assessed as H3 antagonist, later found to be an agonist, shows functional selectivity, activating some G-protein coupled pathways but not others)[12]
- Imbutamine (also H4 agonist)
- Immepip
- Imetit
- Immethridine
- Methimepip
- Proxyfan (complex functional selectivity; partial agonist effects on cAMP inhibition and MAPK activity, antagonist on histamine release, and inverse agonist on arachidonic acid release)
Antagonists
These include:[13]
- A-304121 (No tolerance formation, silent antagonist)[14]
- A-349,821[15]
- ABT-239
- Betahistine (also weak H1 agonist)
- Burimamide (also weak H2 antagonist)
- Ciproxifan
- Clobenpropit (also H4 antagonist)
- Conessine
- Failproxifan (No tolerance formation)
- Impentamine
- Iodophenpropit
- Irdabisant
- Pitolisant
- Thioperamide (also H4 antagonist)
- VUF-5681 (4-[3-(1H-Imidazol-4-yl)propyl]piperidine)
Therapeutic potential
The H3-receptor is a promising potential therapeutical target for many (cognitive) disorders that are caused by a histaminergic H3R dysfunction, because it is linked to the central nervous system and its regulation of other neurotransmitters.[6][16][17] Examples of such disorders are: sleep disorders (including narcolepsy), Tourette syndrome, Parkinson, OCD, ADHD, ASS and drug addictions.[6][17]
This receptor has been proposed as a target for treating sleep disorders.[18] The receptor has also been proposed as a target for treating neuropathic pain.[19]
Because of its ability to modulate other neurotransmitters, H3 receptor ligands are being investigated for the treatment of numerous neurological conditions, including obesity (because of the histamine/orexinergic system interaction), movement disorders (because of H3 receptor-modulation of dopamine and GABA in the basal ganglia), schizophrenia and ADHD (again because of dopamine modulation) and research is underway to determine whether H3 receptor ligands could be useful in modulating wakefulness (because of effects on noradrenaline, glutamate and histamine).[20][7]
There is also evidence that the H3-receptor plays an important role in Tourette syndrome.[21] Mouse-models and other research demonstrated that reducing histamine concentration in the H3R causes tics, but adding histamine in the striatum decreases the symptoms.[22][23][24] The interaction between histamine (H3-receptor) and dopamine as well as other neurotransmitters is an important underlying mechanism behind the disorder.[25]
History
- 1983 The H3 receptor is pharmacologically identified.[26]
- 1988 H3 receptor found to mediate inhibition of serotonin release in rat brain cortex.[27]
- 1997 H3 receptors shown to modulate ischemic norepinephrine release in animals.[28]
- 1999 H3 receptor cloned[29]
- 2000 H3 receptors called "new frontier in myocardial ischemia"[30]
- 2002 H3(-/-) mice (mice that do not have this receptor)[31]
See also
- Antihistamine – histamine receptor antagonists
- H3-receptor antagonist
- Histamine H1-receptor
- Histamine H2-receptor
- Histamine H4-receptor
References
- GRCh38: Ensembl release 89: ENSG00000101180 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000039059 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- West RE, Zweig A, Shih NY, Siegel MI, Egan RW, Clark MA (Nov 1990). "Identification of two H3-histamine receptor subtypes" (abstract). Molecular Pharmacology. 38 (5): 610–3. PMID 2172771.
- Rapanelli, Maximiliano. “The Magnificent Two: Histamine and the H3 Receptor as Key Modulators of Striatal Circuitry.” Progress in Neuro-Psychopharmacology and Biological Psychiatry 73 (February 2017): 36–40
- Sadek, Bassem, Ali Saad, Adel Sadeq, Fakhreya Jalal, and Holger Stark. “Histamine H3 Receptor as a Potential Target for Cognitive Symptoms in Neuropsychiatric Diseases.” Behavioural Brain Research 312 (October 2016): 415–430
- "InterPro: IPR003980 Histamine H3 receptor". InterPro. European Bioinformatics Institute.
- Attoub S, Moizo L, Sobhani I, Laigneau JP, Lewin MJ, Bado A (Jun 2001). "The H3 receptor is involved in cholecystokinin inhibition of food intake in rats". Life Sciences. 69 (4): 469–78. doi:10.1016/S0024-3205(01)01138-9. PMID 11459437.
- Bakker RA (Oct 2004). "Histamine H3-receptor isoforms". Inflammation Research. 53 (10): 509–16. doi:10.1007/s00011-004-1286-9. PMID 15597144. S2CID 9630188.
- Rouleau A, Héron A, Cochois V, Pillot C, Schwartz JC, Arrang JM (Sep 2004). "Cloning and expression of the mouse histamine H3 receptor: evidence for multiple isoforms". Journal of Neurochemistry. 90 (6): 1331–8. doi:10.1111/j.1471-4159.2004.02606.x. PMID 15341517. S2CID 29078902.
- Krueger KM, Witte DG, Ireland-Denny L, et al. (July 2005). "G protein-dependent pharmacology of histamine H3 receptor ligands: evidence for heterogeneous active state receptor conformations". J. Pharmacol. Exp. Ther. 314 (1): 271–81. doi:10.1124/jpet.104.078865. PMID 15821027. S2CID 20470970.
- Tedford CE, Phillips JG, Gregory R, Pawlowski GP, Fadnis L, Khan MA, Ali SM, Handley MK, Yates SL (May 1999). "Development of trans-2-[1H-imidazol-4-yl] cyclopropane derivatives as new high-affinity histamine H3 receptor ligands" (abstract). The Journal of Pharmacology and Experimental Therapeutics. 289 (2): 1160–8. PMID 10215700.
- Pan, Jia Bao; Yao, Betty B.; Miller, Thomas R.; Kroeger, Paul E.; Bennani, Youssef L.; Komater, Victoria A.; Esbenshade, Timothy A.; Hancock, Arthur A.; Decker, Michael W.; Fox, Gerard B. (2006-08-29). "Evidence for tolerance following repeated dosing in rats with ciproxifan, but not with A-304121". Life Sciences. 79 (14): 1366–1379. doi:10.1016/j.lfs.2006.04.002. ISSN 0024-3205. PMID 16730751.
- Esbenshade TA, Fox GB, Krueger KM, et al. (September 2004). "Pharmacological and behavioral properties of A-349821, a selective and potent human histamine H3 receptor antagonist". Biochem. Pharmacol. 68 (5): 933–45. doi:10.1016/j.bcp.2004.05.048. PMID 15294456.
- Bolam, J. Paul, and Tommas J. Ellender. “Histamine and the Striatum.” Neuropharmacology 106 (July 2016): 74–84
- Sadek, Bassem, Ali Saad, Adel Sadeq, Fakhreya Jalal, and Holger Stark. “Histamine H3 Receptor as a Potential Target for Cognitive Symptoms in Neuropsychiatric Diseases.” Behavioural Brain Research 312 (October 2016): 415–430
- Passani MB, Lin JS, Hancock A, Crochet S, Blandina P (Dec 2004). "The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders". Trends in Pharmacological Sciences. 25 (12): 618–25. doi:10.1016/j.tips.2004.10.003. PMID 15530639.
- Medhurst SJ, Collins SD, Billinton A, Bingham S, Dalziel RG, Brass A, Roberts JC, Medhurst AD, Chessell IP (Aug 2008). "Novel histamine H3 receptor antagonists GSK189254 and GSK334429 are efficacious in surgically-induced and virally-induced rat models of neuropathic pain". Pain. 138 (1): 61–9. doi:10.1016/j.pain.2007.11.006. PMID 18164820. S2CID 43724064.
- Leurs R, Bakker RA, Timmerman H, de Esch IJ (Feb 2005). "The histamine H3 receptor: from gene cloning to H3 receptor drugs". Nature Reviews. Drug Discovery. 4 (2): 107–20. doi:10.1038/nrd1631. PMID 15665857. S2CID 32781560.
- Cox, Joanna H., Stefano Seri, and Andrea E. Cavanna. “Histaminergic Modulation in Tourette Syndrome.” Expert Opinion on Orphan Drugs 4, no. 2 (February 1, 2016): 205–213
- Bolam, J. Paul, and Tommas J. Ellender. “Histamine and the Striatum.” Neuropharmacology 106 (July 2016): 74–84
- Rapanelli, Maximiliano, Luciana Frick, Haruhiko Bito, and Christopher Pittenger. “Histamine Modulation of the Basal Ganglia Circuitry in the Development of Pathological Grooming.” Proceedings of the National Academy of Sciences (June 5, 2017): 6599–6604
- Rapanelli, Maximiliano, and Christopher Pittenger. “Histamine and Histamine Receptors in Tourette Syndrome and Other Neuropsychiatric Conditions.” Neuropharmacology 106 (July 2016): 85–90
- Baldan, Lissandra Castellan, Kyle A. Williams, Jean-Dominique Gallezot, Vladimir Pogorelov, Maximiliano Rapanelli, Michael Crowley, George M. Anderson, et al. “Histidine Decarboxylase Deficiency Causes Tourette Syndrome: Parallel Findings in Humans and Mice.” Neuron 81, no. 1 (January 8, 2014): 77–90
- Arrang JM, Garbarg M, Schwartz JC (Apr 1983). "Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor". Nature. 302 (5911): 832–7. Bibcode:1983Natur.302..832A. doi:10.1038/302832a0. PMID 6188956. S2CID 4302564.
- Schlicker E, Betz R, Göthert M (May 1988). "Histamine H3 receptor-mediated inhibition of serotonin release in the rat brain cortex". Naunyn-Schmiedeberg's Archives of Pharmacology. 337 (5): 588–90. doi:10.1007/BF00182737. PMID 3412497. S2CID 24168192.
- Hatta E, Yasuda K, Levi R (Nov 1997). "Activation of histamine H3 receptors inhibits carrier-mediated norepinephrine release in a human model of protracted myocardial ischemia" (abstract). The Journal of Pharmacology and Experimental Therapeutics. 283 (2): 494–500. PMID 9353362.
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, Erlander MG (Jun 1999). "Cloning and functional expression of the human histamine H3 receptor". Molecular Pharmacology. 55 (6): 1101–7. doi:10.1124/mol.55.6.1101. PMID 10347254. S2CID 25542667.
- Levi R, Smith NC (Mar 2000). "Histamine H(3)-receptors: a new frontier in myocardial ischemia" (abstract). The Journal of Pharmacology and Experimental Therapeutics. 292 (3): 825–30. PMID 10688593.
- Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, Wu Y, Lee DH, Yanai K, Sakurai E, Watanabe T, Liu C, Chen J, Barbier AJ, Turek FW, Fung-Leung WP, Lovenberg TW (Aug 2002). "Behavioral characterization of mice lacking histamine H(3) receptors". Molecular Pharmacology. 62 (2): 389–97. doi:10.1124/mol.62.2.389. PMID 12130692. S2CID 25583387.
Further reading
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL (Sep 1997). "International Union of Pharmacology. XIII. Classification of histamine receptors". Pharmacological Reviews. 49 (3): 253–78. PMID 9311023.
- Malinowska B, Godlewski G, Schlicker E (Jun 1998). "Histamine H3 receptors--general characterization and their function in the cardiovascular system". Journal of Physiology and Pharmacology. 49 (2): 191–211. PMID 9670104.
- Leurs R, Hoffmann M, Wieland K, Timmerman H (Jan 2000). "H3 receptor gene is cloned at last". Trends in Pharmacological Sciences. 21 (1): 11–2. doi:10.1016/S0165-6147(99)01411-X. PMID 10637648.
- Leurs R, Bakker RA, Timmerman H, de Esch IJ (Feb 2005). "The histamine H3 receptor: from gene cloning to H3 receptor drugs". Nature Reviews. Drug Discovery. 4 (2): 107–20. doi:10.1038/nrd1631. PMID 15665857. S2CID 32781560.
- Esbenshade TA, Fox GB, Cowart MD (Apr 2006). "Histamine H3 receptor antagonists: preclinical promise for treating obesity and cognitive disorders". Molecular Interventions. 6 (2): 77–88, 59. doi:10.1124/mi.6.2.5. PMID 16565470.
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, Erlander MG (Jun 1999). "Cloning and functional expression of the human histamine H3 receptor". Molecular Pharmacology. 55 (6): 1101–7. doi:10.1124/mol.55.6.1101. PMID 10347254. S2CID 25542667.
- Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K (Dec 2000). "Molecular cloning and characterization of a new human histamine receptor, HH4R". Biochemical and Biophysical Research Communications. 279 (2): 615–20. doi:10.1006/bbrc.2000.4008. PMID 11118334.
- Cogé F, Guénin SP, Audinot V, Renouard-Try A, Beauverger P, Macia C, Ouvry C, Nagel N, Rique H, Boutin JA, Galizzi JP (Apr 2001). "Genomic organization and characterization of splice variants of the human histamine H3 receptor". The Biochemical Journal. 355 (Pt 2): 279–88. doi:10.1042/0264-6021:3550279. PMC 1221737. PMID 11284713.
- Silver RB, Poonwasi KS, Seyedi N, Wilson SJ, Lovenberg TW, Levi R (Jan 2002). "Decreased intracellular calcium mediates the histamine H3-receptor-induced attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings". Proceedings of the National Academy of Sciences of the United States of America. 99 (1): 501–6. Bibcode:2002PNAS...99..501S. doi:10.1073/pnas.012506099. PMC 117589. PMID 11752397.
- Wiedemann P, Bönisch H, Oerters F, Brüss M (Apr 2002). "Structure of the human histamine H3 receptor gene (HRH3) and identification of naturally occurring variations". Journal of Neural Transmission. 109 (4): 443–53. doi:10.1007/s007020200036. PMID 11956964. S2CID 32434148.
- Wellendorph P, Goodman MW, Burstein ES, Nash NR, Brann MR, Weiner DM (Jun 2002). "Molecular cloning and pharmacology of functionally distinct isoforms of the human histamine H(3) receptor". Neuropharmacology. 42 (7): 929–40. doi:10.1016/S0028-3908(02)00041-2. PMID 12069903. S2CID 54326678.
- Lozeva V, Tuomisto L, Tarhanen J, Butterworth RF (Oct 2003). "Increased concentrations of histamine and its metabolite, tele-methylhistamine and down-regulation of histamine H3 receptor sites in autopsied brain tissue from cirrhotic patients who died in hepatic coma". Journal of Hepatology. 39 (4): 522–7. doi:10.1016/S0168-8278(03)00353-2. PMID 12971961.
- Lippert U, Artuc M, Grützkau A, Babina M, Guhl S, Haase I, Blaschke V, Zachmann K, Knosalla M, Middel P, Krüger-Krasagakis S, Henz BM (Jul 2004). "Human skin mast cells express H2 and H4, but not H3 receptors". The Journal of Investigative Dermatology. 123 (1): 116–23. doi:10.1111/j.0022-202X.2004.22721.x. PMID 15191551.
External links
- "Histamine Receptors: H3". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
- H3+receptors at the U.S. National Library of Medicine Medical Subject Headings (MeSH)