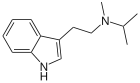
Methylisopropyltryptamine
N-Methyl-N-isopropyltryptamine (MiPT) is a psychedelic tryptamine, closely related to DMT, DiPT and Miprocin.
| |||
| Legal status | |||
|---|---|---|---|
| Legal status |
| ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| ChemSpider |
| ||
| UNII | |||
| ChEMBL | |||
| Chemical and physical data | |||
| Formula | C14H20N2 | ||
| Molar mass | 216.328 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| (verify) | |||
Chemistry
MiPT base, unlike many other tryptamines in their freebase form, does not decompose rapidly in the presence of light or oxygen.
In August 2019, Chadeayne et al. solved the crystal structure of MiPT fumarate.[1] Its systematic name is [2-(1H-indol-3-yl)ethyl](methyl)propan-2-ylazanium 3-carboxyprop-2-enoate. The salt consists of a protonated tryptammonium cation and a 3-carboxyacrylate (hydrogen fumarate) anion in the asymmetric unit.
Effects
MiPT is said to emphasize psychedelic/entheogenic effects over sensory/hallucinogenic activity. Users report strong mental effects, but few perceptual alterations.
Hyper sensitivity to sound as well
Legality
Sweden's public health agency suggested classifying MiPT as a hazardous substance, on May 15, 2019.[2]
In the United States MiPT is considered a schedule 1 controlled substance as a positional isomer of Diethyltryptamine (DET). MiPT is specifically mentioned by name in the DEA Controlled Substance Orange Book. [3]
See also
References
- Chadeayne AR, Pham DN, Golen JA, Manke DR (September 2019). "N-methyl derivatives of DMT and psilocin". Acta Crystallographica Section E. 75 (Pt 9): 1316–1320. doi:10.1107/S2056989019011253. PMC 6727059. PMID 31523457.
- "Folkhälsomyndigheten föreslår att 20 ämnen klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 15 May 2019.
- "Orange Book - List of Controlled Substances and Regulated Chemicals" (PDF). Drug Enforcement Administration. Archived (PDF) from the original on 6 March 2023.
External links
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
|