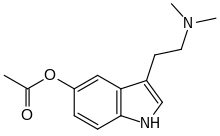
O-Acetylbufotenine
O-Acetylbufotenine (5-AcO-DMT, bufotenine acetate) is a tryptamine derivative which produces psychedelic-appropriate responding in animal studies. It is an acylated derivative of bufotenine with higher lipophilicity that allows it to cross the blood–brain barrier; once inside the brain, it is metabolised to bufotenine.[1][2][3] It also acts directly as an agonist at 5-HT1A and 5-HT1D receptors.[4]
 | |
| Clinical data | |
|---|---|
| Other names | 3-(2-(dimethylamino)ethyl)-1H-indol-5-yl acetate; 5-Acetoxy-DMT; 5-Acetoxy-N,N-dimethyltryptamine; 5-AcO-DMT; 3-(2-(Dimethylamino)ethyl]-1H-indol-5-yl acetate |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H18N2O2 |
| Molar mass | 246.310 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Shulgin & Shulgin. TiHKAL #19. 5-HO-DMT
- Gessner PK, Dankova JB (January 1975). "Brain Bufotenine from Administered Acetylbufotenine: Comparison of Its Tremorgenic Activity with That of N,N-Dimethyltryptamine and 5-Methoxy-N,N-Dimethyltryptamine". Pharmacologist. 17 (2): 259.
- Winter JC, Amorosi DJ, Rice KC, Cheng K, Yu AM (September 2011). "Stimulus control by 5-methoxy-N,N-dimethyltryptamine in wild-type and CYP2D6-humanized mice". Pharmacology, Biochemistry, and Behavior. 99 (3): 311–5. doi:10.1016/j.pbb.2011.05.015. PMC 3129464. PMID 21624387.
- Glennon RA, Hong SS, Bondarev M, Law H, Dukat M, Rakhi S, Power P, Fan E, Kinneau D, Kamboj R, Teitler M, Herrick-Davis K, Smith C (January 1996). "Binding of O-alkyl derivatives of serotonin at human 5-HT1D beta receptors". Journal of Medicinal Chemistry. 39 (1): 314–22. doi:10.1021/jm950498t. PMID 8568822.
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.