Gentamicin
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˌdʒɛntəˈmaɪsən/ |
| Trade names | Cidomycin, Genticyn, Garamycin, others |
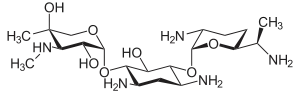
IUPAC name
| |
| Clinical data | |
| Drug class | Aminoglycoside antibiotic |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | IV, eye drop, IM, topical, ear drop |
| Defined daily dose | 240 mg[2] |
| External links | |
| AHFS/Drugs.com | Systemic: Monograph Topical: Monograph Eye: Monograph |
| MedlinePlus | a682275 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | limited bioavailability by mouth |
| Protein binding | 0–10% |
| Elimination half-life | 2 h |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C21H43N5O7 |
| Molar mass | 477.603 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Gentamicin, sold under brand name Garamycin among others, is an antibiotic used to treat several types of bacterial infections.[3] This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others.[3] It is not effective for gonorrhea or chlamydia infections.[3] It can be given intravenously, by injection into a muscle, or topically.[3] Topical formulations may be used in burns or for infections of the outside of the eye.[4] In the developed world, it is often only used for two days until bacterial cultures determine what specific antibiotics the infection is sensitive to.[5] The dose required should be monitored by blood testing.[3]
Gentamicin can cause inner ear problems and kidney problems.[3] The inner ear problems can include problems with balance and hearing loss.[3] These problems may be permanent.[3] If used during pregnancy, it can cause harm to the developing baby.[3] However, it appears to be safe for use during breastfeeding.[6] Gentamicin is a type of aminoglycoside.[3] It works by disrupting the ability of the bacteria to make proteins, which typically kills the bacteria.[3]
Gentamicin was patented in 1962 and approved for medical use in 1964.[7] It is made from the bacterium Micromonospora purpurea.[3] It is on the World Health Organization's List of Essential Medicines.[8] The World Health Organization classifies gentamicin as critically important for human medicine.[9] It is available as a generic medication.[10] The injectable's wholesale cost in the developing world in 2014 was between US$0.05 and US$0.58 per mL.[11] In the UK it costs the NHS around £10 for five vials of 5 mg, as of 2021.[12]
Medical uses
Gentamicin is active against a wide range of bacterial infections, mostly Gram-negative bacteria including Pseudomonas, Proteus, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Serratia, and the Gram-positive Staphylococcus.[13] Gentamicin is used in the treatment of respiratory tract infections, urinary tract infections, blood, bone and soft tissue infections of these susceptible bacteria.[14] It is in the 'access' group of the WHO AWaRe Classification.[15]
There is insufficient evidence to support gentamicin as the first line treatment of Neisseria gonorrhoeae infection.[16] Gentamicin is not used for Neisseria meningitidis or Legionella pneumophila bacterial infections (because of the risk of the person going into shock from lipid A endotoxin found in certain Gram-negative organisms). Gentamicin is also useful against Yersinia pestis (responsible for plague), its relatives, and Francisella tularensis (the organism responsible for tularemia often seen in hunters and trappers).[17]
Some Enterobacteriaceae, Pseudomonas spp., Enterococcus spp., Staphylococcus aureus and other Staphylococcus spp. have varying degrees of resistance to gentamicin.[18]
Dosage
The defined daily dose is 240 mg by injection.[2] The dose in adults is often 1 to 1.7 mg/kg every 8 hours by injection.[19] A dose correction is required in those who are obese.[19]
In children the dose is 2.5 mg/kg by injection every 8 hours.[19] Among those in the first week who weight less than 2 kg the dose is 3 mg/kg once per day, otherwise in the first month of life the dose is 5 mg/kg once per day.[20]
Doses are given less frequently in those with kidney problems.[19]
Side effects
Side effects of gentamicin can range from less severe reactions, such as nausea and vomiting, to more severe reactions including:[13]
- Low blood cell counts
- Allergic reactions
- Neuromuscular problems
- Nerve damage (neuropathy)
- Kidney damage (nephrotoxicity)
- Ear disorders (ototoxicity)
Nephrotoxicity and ototoxicity are thought to be dose related with higher doses causing greater chance of toxicity.[13] These two toxicities may have delayed presentation, sometimes not appearing until after completing treatment.[13]
Kidney damage
Kidney damage is a problem in 10-25% of people who receive aminoglycosides, and gentamicin is one of the most nephrotoxic drugs of this class.[21] Oftentimes, acute nephrotoxicity is reversible, but it may be fatal.[13] The risk of nephrotoxicity can be affected by the dose, frequency, duration of therapy, and concurrent use of certain medications, such as NSAIDs, diuretics, cisplatin, ciclosporin, cephalosporins, amphotericin, iodide contrast media, and vancomycin.[21]
Factors that increase risk of nephrotoxicity include:[21]
- Increased age
- Reduced renal function
- Pregnancy
- Hypothyroidism
- Hepatic dysfunction
- Volume depletion
- Metabolic acidosis
- Sodium depletion
Kidney dysfunction is monitored by measuring creatinine in the blood, electrolyte levels, urine output, presence of protein in the urine, and concentrations of other chemicals, such as urea, in the blood.[21]
Inner ear
About 11% of the population who receives aminoglycosides experience damage to their inner ear.[22] The common symptoms of inner ear damage include tinnitus, hearing loss, vertigo, trouble with coordination, and dizziness.[23] Chronic use of gentamicin can affect two areas of the ears. First, damage of the inner ear hair cells can result in irreversible hearing loss. Second, damage to the inner ear vestibular apparatus can lead to balance problems.[23] To reduce the risk of ototoxicity during treatment, it is recommended to stay hydrated.[13]
Factors that increase the risk of inner ear damage include:[13][14]
- Increased age
- High blood uric acid levels
- Kidney dysfunction
- Liver dysfunction
- Higher doses
- Long courses of therapy
- Also taking strong diuretics (e.g., furosemide)
Pregnancy and breastfeeding
Gentamicin is not recommended in pregnancy unless the benefits outweigh the risks for the mother. Gentamicin can cross the placenta and several reports of irreversible bilateral congenital deafness in children have been seen. Intramuscular injection of gentamicin in mothers can cause muscle weakness in the newborn.[14]
The safety and efficacy for gentamicin in nursing mothers has not been established. Detectable gentamicin levels are found in human breast milk and in nursing babies.[14]
Mechanism of action
Gentamicin is a bactericidal antibiotic that works by binding the 30S subunit of the bacterial ribosome, negatively impacting protein synthesis. The primary mechanism of action is generally accepted to work through ablating the ability of the ribosome to discriminate on proper transfer RNA and messenger RNA interactions.[24] Typically, if an incorrect tRNA pairs with an mRNA codon at the aminoacyl site of the ribosome, adenosines 1492 and 1493 are excluded from the interaction and retract, signaling the ribosome to reject the aminoacylated tRNA::Elongation Factor Thermo-Unstable complex.[25] However, when gentamicin binds at helix 44 of the 16S rRNA, it forces the adenosines to maintain the position they take when there is a correct, or cognate, match between aa-tRNA and mRNA.[26] This leads to the acceptance of incorrect aa-tRNAs, causing the ribosome to synthesize proteins with wrong amino acids placed throughout (roughly every 1 in 500).[27] The non-functional, mistranslated proteins misfold and aggregate, eventually leading to death of the bacterium. A secondary mechanism has been proposed based on crystal structures of gentamicin in a secondary binding site at helix 69 of the 23S rRNA, which interacts with helix 44 and proteins that recognize stop codons. At this secondary site, gentamicin is believed to preclude interactions of the ribosome with ribosome recycling factors, causing the two subunits of the ribosome to stay complexed even after translation completes. This creates a pool of inactive ribosomes that can no longer re-initiate and translate new proteins.[28]
Components
Gentamicin is composed of a number of related gentamicin components and fractions which have varying degrees of antimicrobial potency.[29] The main components of gentamicin include members of the gentamicin C complex: gentamicin C1, gentamicin C1a, and gentamicin C2 which compose approximately 80% of gentamicin and have been found to have the highest antibacterial activity. Gentamicin A, B, X, and a few others make up the remaining 20% of gentamicin and have lower antibiotic activity than the gentamicin C complex.[30] The exact composition of a given sample or lot of gentamicin is not well defined, and the level of gentamicin C components or other components in gentamicin may differ from lot-to-lot depending on the gentamicin manufacturer or manufacturing process. Because of this lot-to-lot variability, it can be difficult to study various properties of gentamicin including pharmacokinetics and microorganism susceptibility if there is an unknown combination of chemically related but different compounds.[31]
Contraindications
Gentamicin should not be used if a person has a history of hypersensitivity, such as anaphylaxis, or other serious toxic reaction to gentamicin or any other aminoglycosides.[14] Greater care is required in people with myasthenia gravis and other neuromuscular disorders as there is a risk of worsening weakness.[3]
Special populations
Elderly
In the elderly, renal function should be assessed before beginning therapy as well as during treatment due to a decline in glomerular filtration rate. Gentamicin levels in the body can remain higher for a longer period of time in this population. Gentamicin should be used cautiously in persons with kidney, auditory, vestibular, or neuromuscular dysfunction.[13]
Children
Gentamicin may not be appropriate to use in children, including babies. Studies have shown higher serum levels and a longer half-life in this population.[32] Kidney function should be checked periodically during therapy. Long-term effects of treatment can include hearing loss and balance problems. Hypocalcemia, hypokalemia, and muscle weakness have been reported when used by injection.[13]
History

Gentamicin is produced by the fermentation of Micromonospora purpurea. It was discovered in 1963 by Weinstein, Wagman et al. at Schering Corporation in Bloomfield, N.J. while working with source material (soil samples) provided by Rico Woyciesjes.[33] Subsequently, it was purified and the structures of its three components were determined by Cooper, et al., also at the Schering Corporation. It was initially used as a topical treatment for burns at burn units in Atlanta and San Antonio and was introduced into IV usage in 1971. It remains a mainstay for use in sepsis.
It is synthesized by Micromonospora, a genus of Gram-positive bacteria widely present in the environment (water and soil). To highlight their specific biological origins, gentamicin and other related antibiotics produced by this genus (verdamicin, mutamicin, sisomicin, netilmicin, and retymicin) generally have their spellings ending in ~micin and not in ~mycin.
Research
Gentamicin is also used in molecular biology research as an antibacterial agent in tissue and cell culture, to prevent contamination of sterile cultures. Gentamicin is one of the few heat-stable antibiotics that remain active even after autoclaving, which makes it particularly useful in the preparation of some microbiological growth media.
Society and culture
Cost
The injectable's wholesale cost in the developing world in 2014 was between US$0.05 and US$0.58 per mL.[11]
.svg.png.webp) Gentamicin costs (US)
Gentamicin costs (US).svg.png.webp) Gentamicin prescriptions (US)
Gentamicin prescriptions (US)
References
- 1 2 "Gentamicin Use During Pregnancy". Drugs.com. 28 February 2019. Archived from the original on 1 August 2020. Retrieved 11 February 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 3 July 2020. Retrieved 1 September 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Gentamicin sulfate". The American Society of Health-System Pharmacists. Archived from the original on 16 August 2015. Retrieved 15 August 2015.
- ↑ Bartlett, Jimmy (2013). Clinical Ocular Pharmacology (s ed.). Elsevier. p. 214. ISBN 9781483193915. Archived from the original on 22 December 2015.
- ↑ Moulds, Robert; Jeyasingham, Melanie (October 2010). "Gentamicin: a great way to start". Australian Prescriber (33): 134–135. Archived from the original on 13 March 2011.
- ↑ "Gentamicin use while breastfeeding". Archived from the original on 6 September 2015. Retrieved 15 August 2015.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 507. ISBN 9783527607495. Archived from the original on 20 December 2016. Retrieved 2 March 2019.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ World Health Organization (2019). Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. hdl:10665/312266. ISBN 9789241515528. License: CC BY-NC-SA 3.0 IGO.
- ↑ Burchum, Jacqueline (2014). Lehne's pharmacology for nursing care. Elsevier Health Sciences. p. 1051. ISBN 9780323340267. Archived from the original on 11 March 2016.
- 1 2 "Gentamicin sulfate". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 15 August 2015.
- ↑ "5. Infection". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. pp. 550–551. ISBN 978-0-85711-413-6.
- 1 2 3 4 5 6 7 8 9 "Gentamicin" (PDF). Baxter Corporation. Archived from the original (PDF) on 4 March 2016. Retrieved 2 November 2015.
- 1 2 3 4 5 "Product Monograph" (PDF). Sandoz Canada Inc. Archived (PDF) from the original on 12 April 2015. Retrieved 2 November 2015.
- ↑ Zanichelli, Veronica; Sharland, Michael; Cappello, Bernadette; Moja, Lorenzo; Getahun, Haileyesus; Pessoa-Silva, Carmem; Sati, Hatim; van Weezenbeek, Catharina; Balkhy, Hanan; Simão, Mariângela; Gandra, Sumanth; Huttner, Benedikt (1 April 2023). "The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance". Bulletin of the World Health Organization. 101 (4): 290–296. doi:10.2471/BLT.22.288614. ISSN 0042-9686. Archived from the original on 7 May 2023. Retrieved 17 November 2023.
- ↑ Hathorn E, Dhasmana D, Duley L, Ross JD (September 2014). "The effectiveness of gentamicin in the treatment of Neisseria gonorrhoeae: a systematic review". Systematic Reviews. 3: 104. doi:10.1186/2046-4053-3-104. PMC 4188483. PMID 25239090.
- ↑ Goljan, Edward F. (2011). Rapid Review Pathology (3rd ed.). Philadelphia, Pennsylvania: Elsevier. p. 241. ISBN 978-0-323-08438-3.
- ↑ "Gentamicin spectrum of bacterial susceptibility and Resistance" (PDF). Archived from the original (PDF) on 20 February 2015. Retrieved 15 May 2012.
- 1 2 3 4 "Gentamicin - WikEM". www.wikem.org. Archived from the original on 25 September 2020. Retrieved 5 August 2020.
- ↑ "GENTAMICIN injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 1 September 2020.
- 1 2 3 4 Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ (January 2011). "New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view". Kidney International. 79 (1): 33–45. doi:10.1038/ki.2010.337. PMID 20861826.
- ↑ East JE, Foweraker JE, Murgatroyd FD (May 2005). "Gentamicin induced ototoxicity during treatment of enterococcal endocarditis: resolution with substitution by netilmicin". Heart. 91 (5): e32. doi:10.1136/hrt.2003.028308. PMC 1768868. PMID 15831617.
- 1 2 Selimoglu E (1 January 2007). "Aminoglycoside-induced ototoxicity". Current Pharmaceutical Design. 13 (1): 119–26. doi:10.2174/138161207779313731. PMID 17266591.
- ↑ "DrugBank-Gentamicin". Archived from the original on 4 October 2013.
- ↑ Dao EH, Poitevin F, Sierra RG, Gati C, Rao Y, Ciftci HI, et al. (December 2018). "Structure of the 30S ribosomal decoding complex at ambient temperature". RNA. 24 (12): 1667–1676. doi:10.1261/rna.067660.118. PMC 6239188. PMID 30139800.
- ↑ Wilson DN (January 2014). "Ribosome-targeting antibiotics and mechanisms of bacterial resistance". Nature Reviews. Microbiology. 12 (1): 35–48. doi:10.1038/nrmicro3155. PMID 24336183.
- ↑ Garrett, Roger; Douthwaite, Stephen; Liljas, Andres; Matheson, Alistair; Moore, Peter; Harry, Noller (2000). The Ribosome. ASM Press. pp. 419–429. ISBN 978-1-55581-184-6.
- ↑ Borovinskaya MA, Pai RD, Zhang W, Schuwirth BS, Holton JM, Hirokawa G, et al. (August 2007). "Structural basis for aminoglycoside inhibition of bacterial ribosome recycling". Nature Structural & Molecular Biology. 14 (8): 727–32. doi:10.1038/nsmb1271. PMID 17660832.
- ↑ Weinstein MJ, Wagman GH, Oden EM, Marquez JA (September 1967). "Biological activity of the antibiotic components of the gentamicin complex". Journal of Bacteriology. 94 (3): 789–90. doi:10.1128/JB.94.3.789-790.1967. PMC 251956. PMID 4962848.
- ↑ Vydrin AF (2003). "Component Composition of Gentamicin Sulfate Preparations". Pharmaceutical Chemistry Journal. 37 (8): 448–449. doi:10.1023/a:1027372416983.
- ↑ Isoherranen N, Lavy E, Soback S (June 2000). "Pharmacokinetics of gentamicin C(1), C(1a), and C(2) in beagles after a single intravenous dose". Antimicrobial Agents and Chemotherapy. 44 (6): 1443–7. doi:10.1128/aac.44.6.1443-1447.2000. PMC 89894. PMID 10817690.
- ↑ Sato Y (February 1997). "Pharmacokinetics of antibiotics in neonates". Acta Paediatrica Japonica. 39 (1): 124–31. doi:10.1111/j.1442-200X.1997.tb03569.x. PMID 9124044.
- ↑ Weinstein MJ, Luedemann GM, Oden EM, Wagman GH, Rosselet JP, Marquez JA, et al. (July 1963). "Gentamicin,1a New Antibiotic Complex from Micromonospora". Journal of Medicinal Chemistry. 6 (4): 463–4. doi:10.1021/jm00340a034. PMID 14184912.
External links
- "Gentamicin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 1 August 2020. Retrieved 11 February 2020.
- Dean L (2015). "Gentamicin Therapy and MT-RNR1 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520359. Bookshelf ID: NBK285956. Archived from the original on 26 October 2020. Retrieved 5 February 2020.
| Identifiers: |
|---|