Selexipag
 | |
| Names | |
|---|---|
| Trade names | Uptravi |
| Other names | ACT-293987, NS-304 |
IUPAC name
| |
| Clinical data | |
| Drug class | Prostacyclin receptor agonist[1] |
| Main uses | Pulmonary arterial hypertension (PAH)[2] |
| Side effects | Headache, diarrhea, muscle pain, flushing, rash[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth, intravenous |
| Typical dose | 200 to 1,600 mcg BID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
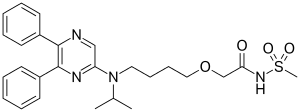
| Formula | C26H32N4O4S |
| Molar mass | 496.63 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Selexipag, sold under the brand name Uptravi, is a medication used to treat pulmonary arterial hypertension (PAH).[2] Generally it is used in people in who an endothelin receptor antagonist and PDE5 inhibitor are not enough.[4] It is taken by mouth or injection into a vein.[2]
Common side effects include headache, diarrhea, muscle pain, flushing, and rash.[3] Other side effects can include pulmonary edema.[3] It should not be used in people with significant coronary artery disease or valvular heart disease.[1] It works by activating the prostacyclin receptor which causes the muscles in blood vessels to relax.[1]
Selexipag was approved for medical use in the United States in 2015 and Europe in 2016.[3][1] In the United States it costs about 20,100 USD a month as of 2021.[5] In the United Kingdom this amount costs the NHS about £3,000.[4]
Medical use
It is used for pulmonary arterial hypertension (AH), which involves abnormally high blood pressure in the arteries of the lungs.[1]
Dosage
It is started at a dose of 200 mcg twice per day, which is than increased up to 1,600 mcg twice per day.[2]
Contraindications
In Europe, use of selexipag together with strong inhibitors of the liver enzyme CYP2C8, such as gemfibrozil, is contraindicated because it increases concentrations of selexipag twofold, and its active metabolite 11-fold, potentially leading to more adverse effects.[6]
Side effects
The adverse effects of selexipag are similar to those of intravenous prostacyclins used for pulmonary arterial hypertension. Common side effects include headache and jaw pain. An increased risk for hyperthyroidism has also been noted in people taking selexipag.
Mechanism of action
Selexipag and its active metabolite, ACT-333679 (or MRE-269, the free carboxylic acid), are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation.[7]
History
The U.S. Food and Drug Administration (FDA) granted selexipag orphan drug status for PAH.[8] It was approved by the FDA on 22 December 2015.[8]
In Europe, the drug was approved in May 2016.[9]
Society and culture
Cost
The price for the drug in the US in 2016 was $160,000 to $170,000 per person before rebates.[10]
References
- 1 2 3 4 5 "Uptravi". Archived from the original on 12 May 2021. Retrieved 11 October 2021.
- 1 2 3 4 5 6 "Uptravi- selexipag tablet, coated Uptravi Titration Pack- selexipag kit". DailyMed. Archived from the original on 30 July 2021. Retrieved 30 July 2021.
- 1 2 3 4 "Selexipag Monograph for Professionals". Drugs.com. Retrieved 11 October 2021.
- 1 2 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 194. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Uptravi Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 3 August 2020. Retrieved 11 October 2021.
- ↑ Information des Bundesamtes für Sicherheit im Gesundheitswesen zu Uptravi (in Deutsch), Österreichisches Bundesamt für Sicherheit im Gesundheitswesen, 2017-06-07
- ↑ Sitbon O, Morrell N (December 2012). "Pathways in pulmonary arterial hypertension: the future is here". European Respiratory Review. 21 (126): 321–7. doi:10.1183/09059180.00004812. PMID 23204120.
- 1 2 New Drug Approved for Rare Lung Disorder. PPN. 23 Dec 2015 Archived 29 December 2015 at the Wayback Machine Has link to GRIPHON study results
- ↑ "Uptravi: Authorisation details". European Medicines Agency. 2016-05-12. Archived from the original on 2018-06-20. Retrieved 2022-03-14.
- ↑ "Actelion sees Uptravi price of $160,000-170,000/patient". Reuters. 2016-01-05. Archived from the original on 2018-11-01. Retrieved 2016-01-06.
External links
| External sites: |
|
|---|---|
| Identifiers: |