Ramelteon
 | |
 | |
| Names | |
|---|---|
| Trade names | Rozerem, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Melatonin receptor agonist[1] |
| Main uses | Trouble falling asleep[2] |
| Side effects | Sleepiness, dizziness, nausea, worsened trouble sleeping[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Addiction risk | None[3] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 8mg HS[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605038 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 1.8% |
| Protein binding | ~82% |
| Metabolism | Liver (CYP1A2-mediated) |
| Elimination half-life | 1–2.6 hours |
| Excretion | Kidney (84%) and fecal (4%) |
| Chemical and physical data | |
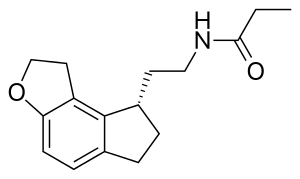
| Formula | C16H21NO2 |
| Molar mass | 259.349 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ramelteon, sold under the brand name Rozerem among others, is a medication used for trouble falling asleep.[2] It is taken by mouth, half an hour before bed.[2] There is no limitation on duration of use; though long term effectiveness is unclear.[4][1]
Common side effects include sleepiness, dizziness, nausea, and worsened trouble sleeping.[2] Other side effects may include anaphylaxis, abnormal thinking, and depression.[2] Effects on pregnancy and breastfeeding are unclear.[5] It is a melatonin receptor agonist and is expected to work similar to melatonin.[1]
Ramelteon was discovered in 1996 and approved for medical use in the United States in 2005.[4][3] It was declined approval in Europe in 2008 due to unclear effectiveness.[1] In the United States it is available as a generic medication and costs about 60 USD per month as of 2021.[6] It is not a controlled substance.[3]
Medical uses
Ramelteon is approved in the United States for the treatment of insomnia characterized by difficulty with sleep onset.[2] It; however, was not approved in Europe due to a lack of benefit.[1]
A 2014 review concluded "ramelteon was found to be beneficial in preventing delirium in medically ill individuals when compared to placebo."[7]
Dosage
It is used at a dose of 8 mg before bed.[2]
Side effects
Ramelteon has not been shown to produce dependence and has shown no potential for abuse, and the withdrawal and rebound insomnia that is typical with GABA modulators is not present in ramelteon.[2]
Six percent of ramelteon-treated patients in clinical trials discontinued due to an adverse event, compared with two percent in the placebo arms. The most frequent adverse events leading to discontinuation were somnolence, dizziness, nausea, fatigue, headache, and insomnia. The United States official Prescribing Information warns of rare cases of anaphylactic reactions, abnormal thinking, worsening of depression or suicidal thinking in patients with pre-existing depression, and decreased testosterone and increased prolactin levels.[8] It also notes that ramelteon is not recommended for use in patients with severe sleep apnea.[8]
In mice treated with ramelteon for two years, increases in liver and testicular tumors were observed, but only at doses at least 20 times greater than the recommended human dose on a milligram/kilogram basis.[8]
Drug interactions
Ramelteon has been evaluated for potential drug interactions with the following medications and showed no significant effects: omeprazole, theophylline, dextromethorphan, and midazolam, digoxin and warfarin. There were no clinically meaningful effects when ramelteon was coadministered with any of these drugs.
A drug interaction study showed that there were no clinically meaningful effects or an increase in adverse events when ramelteon and the SSRI Prozac (fluoxetine) were coadministered. When coadministered with ramelteon, fluvoxamine (strong CYP1A2 inhibitor) increased AUC approximately 190-fold, and the Cmax increased approximately 70-fold, compared to ramelteon administered alone. Ramelteon and fluvoxamine should not be coadministered.[2]
Ramelteon has significant drug–drug interaction with the following drugs: amiodarone, ciprofloxacin, fluvoxamine, ticlopidine.
Ramelteon should be administered with caution in patients taking other CYP1A2 inhibitors, strong CYP3A4 inhibitors such as ketoconazole, and strong CYP2C9 inhibitors such as fluconazole.[2]
Efficacy may be reduced when ramelteon is used in combination with potent CYP enzyme inducers such as rifampin, since ramelteon concentrations may be decreased.
Mechanism of action
Ramelteon is a melatonin receptor agonist with both high affinity for melatonin MT1 and MT2 receptors and selectivity over the MT3 receptor. Ramelteon demonstrates full agonist activity in vitro in cells expressing human MT1 or MT2 receptors, and high selectivity for human MT1 and MT2 receptors compared to the MT3 receptor.[9]
The activity of ramelteon at the MT1 and MT2 receptors is believed to contribute to its sleep-promoting properties, as these receptors, acted upon by endogenous melatonin, are thought to be involved in the maintenance of the circadian rhythm underlying the normal sleep-wake cycle. Ramelteon has no appreciable affinity for the GABA receptor complex or for receptors that bind neuropeptides, cytokines, serotonin, dopamine, noradrenaline, acetylcholine, and opioids. Ramelteon also does not interfere with the activity of a number of selected enzymes in a standard panel.
The major metabolite of ramelteon, M-II, is active and has approximately one tenth and one fifth the binding affinity of the parent molecule for the human MT1 and MT2 receptors, respectively, and is 17–25-fold less potent than ramelteon in in vitro functional assays. Although the potency of M-II at MT1 and MT2 receptors is lower than the parent drug, M-II circulates at higher concentrations than the parent producing 20–100-fold greater mean systemic exposure when compared to ramelteon. M-II has weak affinity for the serotonin 5-HT2B receptor, but no appreciable affinity for other receptors or enzymes. Similar to ramelteon, M-II does not interfere with the activity of a number of endogenous enzymes.
It sleep agent medication that selectively binds to the MT1 and MT2 receptors in the suprachiasmatic nucleus (SCN), instead of binding to GABAA receptors, such as with drugs like zolpidem. Ramelteon does not show any appreciable binding to GABAA receptors, which are associated with anxiolytic, myorelaxant, and amnesic effects.[2]
History
Ramelteon was approved for use in the United States in July 2005.[10]
References
- 1 2 3 4 5 "Ramelteon: Withdrawal of the marketing authorisation application". Archived from the original on 16 October 2021. Retrieved 15 October 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Rozerem- ramelteon tablet, film coated". DailyMed. 28 December 2018. Archived from the original on 26 March 2021. Retrieved 13 April 2020.
- 1 2 3 4 "Ramelteon Monograph for Professionals". Drugs.com. Archived from the original on 25 August 2019. Retrieved 15 October 2021.
- 1 2 Neubauer DN (February 2008). "A review of ramelteon in the treatment of sleep disorders". Neuropsychiatric Disease and Treatment. 4 (1): 69–79. doi:10.2147/ndt.s483. PMC 2515902. PMID 18728808.
- ↑ "Ramelteon (Rozerem) Use During Pregnancy". Drugs.com. Archived from the original on 24 January 2021. Retrieved 15 October 2021.
- ↑ "Ramelteon Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 31 October 2016. Retrieved 15 October 2021.
- ↑ Chakraborti D, Tampi DJ, Tampi RR (March 2015). "Melatonin and melatonin agonist for delirium in the elderly patients". American Journal of Alzheimer's Disease and Other Dementias. 30 (2): 119–29. doi:10.1177/1533317514539379. PMID 24946785.
- 1 2 3 "HIGHLIGHTS OF PRESCRIBING INFORMATION: ROZEREM (ramelteon) tablets, for oral use" (PDF). U.S. Food and Drug Administration. FDA. December 2018. Archived (PDF) from the original on 9 July 2021. Retrieved 2 July 2021.
- ↑ Owen RT (April 2006). "Ramelteon: profile of a new sleep-promoting medication". Drugs of Today. 42 (4): 255–63. doi:10.1358/dot.2006.42.4.970842. PMID 16703122.
- ↑ "Drug Approval Package: Rozerem (Ramelteon) NDA #021782". U.S. Food and Drug Administration (FDA). 20 October 2005. Archived from the original on 31 March 2021. Retrieved 13 April 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |