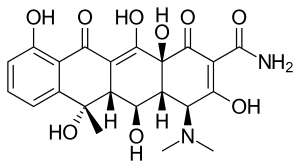
Oxytetracycline
 | |
 | |
| Names | |
|---|---|
| Trade names | Terramycin |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, topical (eye drop) |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Elimination half-life | 6–8 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C22H24N2O9 |
| Molar mass | 460.439 g·mol−1 |
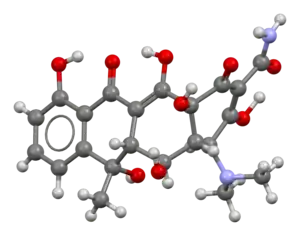
| 3D model (JSmol) | |
| Melting point | 181 to 182 °C (358 to 360 °F) |
SMILES
| |
InChI
| |
Oxytetracycline is an antibiotic used to treat infections such as Chlamydia, including trachoma; Mycoplasma; and rickettsia.[1][2] It is also used for acne and rosacea.[1] It is taken by mouth.[1] A combination formulation is also available as an eye ointment.[3]
Common side effects may include diarrhea, headache, allergic reactions, sun sensitivity, and vomiting.[1] Use is generally not recommended in those under the age of 12 years due to concerns of staining of teeth.[1] Use during pregnancy may harm the baby.[1] It is a broad-spectrum tetracycline antibiotic.[4] It works by blocking the ability of bacteria to produce proteins.[4]
Oxytetracycline was patented in 1949 and came into commercial use in 1950.[5] It is on the World Health Organization's List of Essential Medicines as an alternative to tetracycline.[6] In the United Kingdom a week of treatment costs the NHS about £1.40 as of 2023.[7]
Medical uses
Oxytetracycline, like other tetracyclines, is used to treat many infections, both common and rare (see Tetracycline antibiotics group). Its better absorption profile makes it preferable to tetracycline for moderately severe acne at a dosage of 250–500 mg four times a day for usually six to eight weeks at a time, but alternatives should be sought if no improvement occurs by three months.[8]
It is sometimes used to treat spirochaetal infections, clostridial wound infection and anthrax in patients sensitive to penicillin. Oxytetracycline is used to treat infections of the respiratory and urinary tracts, skin, ear, eye and gonorrhoea, although its use for such purposes has declined in recent years due to large increases in bacterial resistance to this class of drugs. The drug is particularly useful when penicillins and/or macrolides cannot be used due to allergy. It may be used to treat Legionnaire's disease as a substitute for a macrolide or quinolone.
Oxytetracycline is especially valuable in treating nonspecific urethritis, Lyme disease, brucellosis, cholera, typhus, tularaemia. and infections caused by Chlamydia, Mycoplasma and Rickettsia. Doxycycline is now preferred to oxytetracycline for many of these indications because it has improved pharmacologic features.
Dosage
The standard dose is 250–500 mg six-hourly by mouth.[1] In particularly severe infections, this dose may be increased accordingly. Occasionally, oxytetracycline is given by intramuscular injection or topically in the form of creams, ophthalmic ointments or eye drops.
Side effects
Side effects are mainly gastrointestinal and photosensitive allergic reactions common to the tetracycline antibiotics group. It can also damage calcium-rich organs, such as teeth and bones, although this is very rare. It sometimes causes nasal cavities to erode; quite commonly, the BNF suggests, because of this, tetracyclines should not be used to treat pregnant or lactating women and children under 12 except in certain conditions where it has been approved by a specialist because there are no obvious substitutes. Candidiasis (thrush) is not uncommon following treatment with broad-spectrum antibiotics.
History
It was first found near Pfizer laboratories in a soil sample yielding the soil actinomycete, Streptomyces rimosus by Finlay et al. In 1950, a group at Pfizer led by Francis A. Hochstein, working in a loose collaboration with the Harvard organic chemist Robert B Woodward, worked out the chemical structure of oxytetracycline, enabling Pfizer to mass-produce the drug under the trade name Terramycin. This discovery was a major advancement in tetracycline research and paved the way for the discovery of an oxytetracycline derivative, doxycycline, which is one of the most popularly used antibiotics today.
Biosynthesis
Oxytetracycline belongs to a structurally diverse class of aromatic polyketide antibiotics produced by Streptomyces via type II polyketide synthases (PKSs) which are also known as bacterial aromatic polyketides.[9] Other compounds produced via type II PKSs are important bioactive compounds which span from anticancer agents doxorubicin to antibiotics such as tetracycline. The biosynthesis of oxytetracycline can be broken down into three general portions;[10] first is the formation of an amidated polyketide backbone with minimal PKS's, second is the cyclization of the polyketide backbone and finally, the formation of anhydrotetracycline—a shared intermediate with tetracycline—to produce oxytetracycline.
The biosynthesis of oxytetracycline begins with the utilization of PKS enzymes ketosynthase (KS), the chain length factor (CLF), the acyl carrier protein (ACP), and an acyltransferase (encoded as OxyA, OxyB, OxyC and OxyP in the oxytetracycline gene cluster)[11] to catalyze the extension of the malonamyl-CoA starting unit with eight malonyl-CoA extender units. The process of elongating the polypeptide skeleton occurs through a series of Claisen-like decarboxylation reactions until the linear tetracyclic skeleton is formed.[12] Thus, minimal PKS's form a completed amidated polyketide backbone without any additional post-synthase tailoring enzymes (Figure 1).
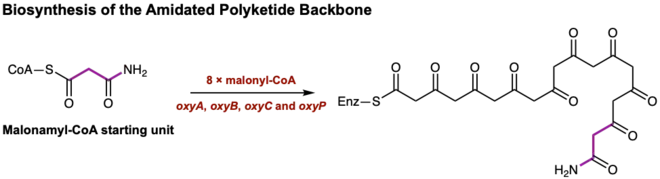
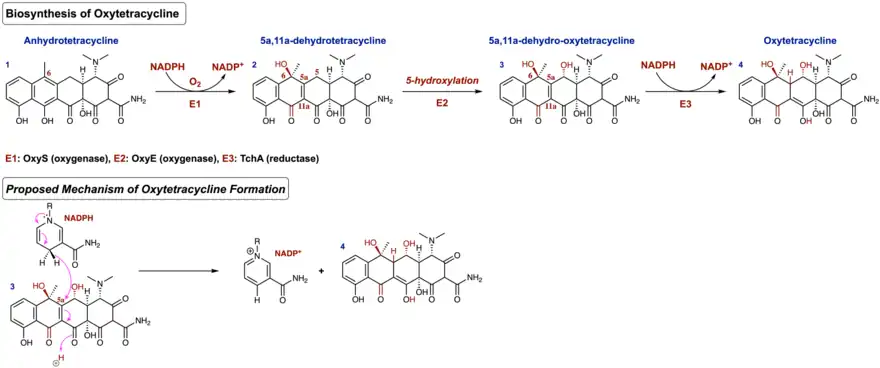
Following the formation of the linear tetracyclic skeleton, four successive cyclization reactions must occur in a regioselective manner to produce the aromatic natural product known as pretetramid—a common precursor to both oxytetracycline and other tetracycline antibiotics.[13] In the oxytetracycline gene cluster, these enzymes are encoded as OxyK (aromatase), OxyN (cyclase), and OxyI (cyclase).[14] Formation of pretetramid allows for one of the most important intermediates en route to the biosynthesis of oxytetracycline; this is the generation of anhydrotetracycline.[15] Anhydrotetracycline contains the first functionalized A ring in this biosynthetic pathway.
After the formation of anhydrotetracycline, ATC monooxygenase (OxyS) oxidizes the C-6 position in an enantioselective manner in the presence of the cofactor NADPH and atmospheric oxygen to produce 5a,11a-dehydrotetracycline.[16] Next, a hydroxylation occurs at the C-5 position of 5a,11a-dehydrotetracycline via the oxygenase encoded as OxyE in the oxytetracycline gene cluster. This produces the intermediate 5a,11a-dehydro-oxytetracycline. However, the exact mechanism of this step remains to be unclear. The final step of this biosynthesis occurs through the reduction of a double bond in the α, β—unsaturated ketone of 5a,11a-dehydro-oxytetracycline. In this final step, the cofactor NADPH is employed by TchA (reductase) as the reducing agent. Upon reduction, the enol form is favored due to conjugation, thus producing the aromatic polyketide oxytetracycline. Figure 2 shows the biosynthesis as described above, as well as an arrow-pushing mechanism of NADPH being used as the final cofactor in the biosynthesis of oxytetracycline.
Other animals
Oxytetracycline is used to control the outbreak of American foulbrood and European foulbrood in honeybees.
Oxytetracycline can also be used to correct breathing disorders in livestock. It is administered in a powder or through an intramuscular injection. American livestock producers apply oxytetracycline to livestock feed to prevent diseases and infections in cattle and poultry. The antibiotic is partially absorbed in the gastrointestinal tract of the animal and the remaining is deposited in manure. Researchers at the Agricultural Research Service studied the breakdown of oxytetracycline in manure depending on various environmental conditions. They found the breakdown slowed with increased saturation of the manure and concluded this was a result of decreased oxygen levels.[17] This research helps producers understand the effects of oxytetracycline in animal feed on the environment, bacteria, and antimicrobial resistance.
Oxytetracycline is used to mark fish which are released and later recaptured. The oxytetracycline interferes with bone deposition, leaving a visible mark on growing bones.
Oxytetracycline has also been formulated as a broad-spectrum anti-infective for fish under the name Terramycin 200 (TM200).[18] It is used to control certain diseases that adversely affect salmonids, catfish, and lobsters.
References
- 1 2 3 4 5 6 7 "Oxytetracycline". NICE. BNF. Archived from the original on 10 September 2023. Retrieved 8 September 2023.
- ↑ "eEML - Electronic Essential Medicines List". list.essentialmeds.org. Archived from the original on 4 June 2023. Retrieved 8 September 2023.
- ↑ "DailyMed - TERRAMYCIN- oxytetracycline hydrochloride and polymyxin b sulfate ointment". dailymed.nlm.nih.gov. Archived from the original on 24 January 2022. Retrieved 8 September 2023.
- 1 2 "DailyMed - terramycin- Oxytetracycline Hydrochloride capsule". dailymed.nlm.nih.gov. Archived from the original on 28 September 2022. Retrieved 8 September 2023.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 9783527607495.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ "Oxytetracycline Medicinal forms". NICE. BNF. Archived from the original on 10 September 2023. Retrieved 8 September 2023.
- ↑ British National Formulary 45 March 2003
- ↑ Talapatra SK, Talapatra B (2015). Polyketide Pathway. Biosynthesis of Diverse Classes of Aromatic Compounds. In: Chemistry of Plant Natural Products. Springer, Berlin, Heidelberg. doi:10.1007/978-3-642-45410-3_14. ISBN 978-3-642-45410-3.
- ↑ Pickens LB, Tang Y (September 2010). "Oxytetracycline biosynthesis". The Journal of Biological Chemistry. 285 (36): 27509–15. doi:10.1074/jbc.R110.130419. PMC 2934616. PMID 20522541.
- ↑ Zhang W, Ames BD, Tsai SC, Tang Y (April 2006). "Engineered biosynthesis of a novel amidated polyketide, using the malonamyl-specific initiation module from the oxytetracycline polyketide synthase". Applied and Environmental Microbiology. 72 (4): 2573–80. Bibcode:2006ApEnM..72.2573Z. doi:10.1128/AEM.72.4.2573-2580.2006. PMC 1449064. PMID 16597959.
- ↑ Tang Y, Tsai SC, Khosla C (October 2003). "Polyketide chain length control by chain length factor". Journal of the American Chemical Society. 125 (42): 12708–9. doi:10.1021/ja0378759. PMID 14558809.
- ↑ McCormick JR, Johnson S (June 1, 1963). "Biosynthesis of the Tetracyclines. V. Naphthacenic Precursors". J. Am. Chem. Soc. 85 (11): 1692–1694. doi:10.1021/ja00894a037.
- ↑ Zhang W, Watanabe K, Wang CC, Tang Y (August 2007). "Investigation of early tailoring reactions in the oxytetracycline biosynthetic pathway". The Journal of Biological Chemistry. 282 (35): 25717–25. doi:10.1074/jbc.M703437200. PMID 17631493.
- ↑ Anhydrotetracycline Archived 2021-10-17 at the Wayback Machine.
- ↑ Peric-Concha N, Borovicka B, Long PF, Hranueli D, Waterman PG, Hunter IS (November 2005). "Ablation of the otcC gene encoding a post-polyketide hydroxylase from the oxytetracyline biosynthetic pathway in Streptomyces rimosus results in novel polyketides with altered chain length". The Journal of Biological Chemistry. 280 (45): 37455–60. doi:10.1074/jbc.M503191200. PMID 16148009.
- ↑ Perry A (March 4, 2010). "Assessing Antibiotic Breakdown in Manure". Agricultural Research Service, U.S. Department of Agriculture. Archived from the original on 27 September 2015. Retrieved 5 February 2023.
- ↑ "Terramycin 200 Antibiotic for Disease Control in Fish Farming". Syndel. Archived from the original on 2019-12-12. Retrieved 2019-12-12.
External links
| Identifiers: |
|---|