MDMAI
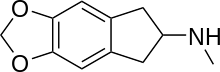
5,6-Methylenedioxy-N-methyl-2-aminoindane (MDMAI), is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in animals and a putative entactogen in humans.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H13NO2 |
| Molar mass | 191.230 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Chemistry
MDMAI can be thought of as a cyclised analogue of MDMA where the alpha-methyl carbon of the alkylamino side chain has been joined back round to the 6-position of the aromatic ring to form an indane ring system. This changes the core structure of the molecule from phenethylamine to aminoindane, and causes the pharmacological properties of the two compounds to be substantially different.[1]
References
- Oberlender R, Nichols DE (1990). "(+)-N-methyl-1-(1,3-benzodioxol-5-yl)-2-butanamine as a discriminative stimulus in studies of 3,4-methylenedioxy-methamphetamine-like behavioral activity". J Pharmacol Exp Ther. 255 (3): 1098–1106. PMID 1979813.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.