MMAI
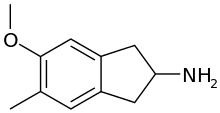
5-Methoxy-6-methyl-2-aminoindane (MMAI) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University.[1] It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) and produces entactogen effects in humans.[1][2][3][4] It has been sold as a designer drug and research chemical online since 2010.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15NO |
| Molar mass | 177.247 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
MMAI has been shown to relieve stress-induced depression in rats more robustly than sertraline,[5] and as a result it has been suggested that SSRAs like MMAI and 4-MTA could be developed as novel antidepressants with a faster onset of therapeutic action and superior efficacy to current antidepressants such as the selective serotonin reuptake inhibitors (SSRIs).[6]
References
- Marona-Lewicka D, Nichols DE (1994). "Behavioral effects of the highly selective serotonin releasing agent 5-methoxy-6-methyl-2-aminoindan". Eur J Pharmacol. 258 (1–2): 1–13. CiteSeerX 10.1.1.688.1895. doi:10.1016/0014-2999(94)90051-5. PMID 7925587.
- Li Q, Murakami I, Stall S, Levy AD, Brownfield MS, Nichols DE, Van de Kar LD (1996). "Neuroendocrine pharmacology of three serotonin releasers: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi) and p-methylthioamphetamine (MTA)". J Pharmacol Exp Ther. 279 (3): 1261–1267. PMID 8968349.
- Rudnick G, Wall SC (1993). "Non-neurotoxic amphetamine derivatives release serotonin through serotonin transporters". Mol. Pharmacol. 43 (2): 271–276. PMID 8429828.
- Luethi D, Kolaczynska KE, Docci L, Krähenbühl S, Hoener MC, Liechti ME (2018). "Pharmacological profile of mephedrone analogs and related new psychoactive substances" (PDF). Neuropharmacology. 15 (134): 4–12. doi:10.1016/j.neuropharm.2017.07.026. PMID 28755886. S2CID 28786127.
- Marona-Lewicka D, Nichols DE (1997). "The Effect of Selective Serotonin Releasing Agents in the Chronic Mild Stress Model of Depression in Rats". Stress. 2 (2): 91–100. doi:10.3109/10253899709014740. PMID 9787258.
- Neuropharmacology; Silveira, R; Nichols, DE; Reyes-Parada, M (1999). "Effects of 5-HT-releasing agents on the extracellullar hippocampal 5-HT of rats. Implications for the development of novel antidepressants with a short onset of action". Neuropharmacology. 38 (7): 1055–1061. doi:10.1016/S0028-3908(99)00023-4. PMID 10428424. S2CID 13714807.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.