Carboprost
 | |
| Names | |
|---|---|
| Trade names | Hemabate, Tham |
IUPAC name
| |
| Clinical data | |
| Drug class | Oxytocic[1] |
| Main uses | Postpartum bleeding, termination of pregnancy[1] |
| Side effects | Diarrhea, headache, nausea, vasodilation, fever, abdominal pain[2][1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intramuscular |
| Typical dose | 250 mcg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600042 |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C21H36O5 |
| Molar mass | 368.514 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Carboprost, sold under the brand name Hemabate among others, is a medication used to treat postpartum bleeding and termination of pregnancy.[1] For postpartum bleeding it is used when oxytocin and ergometrine are not sufficient.[2] For termination it is used in the second trimester when other methods have not worked.[1] It is given by injection into a muscle.[1]
Common side effects include diarrhea, headache, nausea, vasodilation, fever, and abdominal pain.[2][1] Other side effects may include uterine rupture, chest pain, shortness of breath, and ringing in the ears.[2] It is an oxytocic.[1] It works similar to PGF2α by increasing contractions of the uterus.[1]
Carboprost was approved for medical use in the United States in 1979.[1] In the United Kingdom it costs the NHS about £18 per dose as of 2021.[2] This amount in the United States costs about 330 USD.[3]
Medical uses
Used in postpartum hemorrhage caused by uterine atony not controlled by other methods. One study has shown that carboprost tromethamine is more effective than oxytocin in preventing postpartum hemorrhage in high-risk patients undergoing cesarean delivery.[4] Carboprost is also used for the termination of pregnancy in the 2nd trimester.[5]
Unlabeled use:
- Hemorrhagic cystitis
- PID
Dosage
It is often used at a dose of 250 mcg.[1] For bleeding further doses every 15 to 90 minutes may be used.[1] Up to 8 doses may be used for this purpose.[2]
Contraindication
Contraindicated in severe cardiovascular, renal, and hepatic disease. It is also contraindicated in acute Pelvic Inflammatory Disease. Hypersensitivity to carboprost or any of its components is also a contraindication[5] Exert caution in asthmatic patients as carboprost may cause bronchospasm.
Side efects
- diarrhea (most common, may be sudden in onset)
- flushing or hot flashes
- fever
- chills
- nausea/vomiting
Precautions
- asthma
- anemia
- jaundice
- diabetes mellitus
- seizure disorders
- past uterine surgery
Chemistry
Carboprost is supplied with its salt derivative tromethamine in 1 milliliter ampules containing a 250 microgram/milliliter solution of the active drug. The drug must be refrigerated at a temperature between 2 – 8 degrees Celsius.[5]
Synthesis
A significant deactivating metabolic transformation of natural prostaglandins is enzymatic oxidation of the C-15 hydroxyl to the corresponding ketone. This is prevented, with retention of activity, by methylation to give the C-15 tertiary carbinol series.
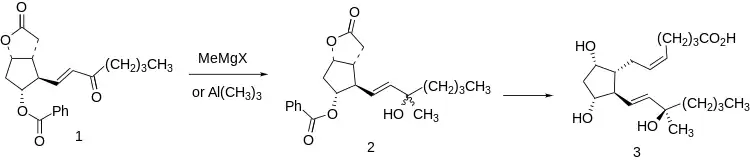
This molecular feature is readily introduced at the stage of the Corey lactone (1) by reaction with methyl Grignard reagent or trimethylaluminium. The resulting mixture of tertiary carbinols (2) is transformed to oxytocic carboprost (3) by standard transformations, including separation of diastereomers, so that the final product is the C-15 analogue. This diastereomer is reputably freeer of porstaglandin side effects than the C-15 (S) isomer.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Carboprost Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 30 December 2021.
- 1 2 3 4 5 6 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 871. ISBN 978-0857114105.
- ↑ "Carboprost Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 January 2021. Retrieved 30 December 2021.
- ↑ Bai J, Sun Q, Zhai H (January 2014). "A comparison of oxytocin and carboprost tromethamine in the prevention of postpartum hemorrhage in high-risk patients undergoing cesarean delivery". Experimental and Therapeutic Medicine. 7 (1): 46–50. doi:10.3892/etm.2013.1379. PMC 3861477. PMID 24348762.
- 1 2 3 Hemabate [Package Insert]. New York, NY: Pharmacia and Upjohn Company; 2014.
- ↑ Yankee EW, Axen U, Bundy GL (September 1974). "Total synthesis of 15-methylprostaglandins". Journal of the American Chemical Society. 96 (18): 5865–76. doi:10.1021/ja00825a027. PMID 4416671.
- ↑ Bundy G, Lincoln F, Nelson N, Pike J, Schneider W (April 1971). "Novel prostaglandin syntheses". Annals of the New York Academy of Sciences. 180 (1): 76–90. Bibcode:1971NYASA.180...76B. doi:10.1111/j.1749-6632.1971.tb53186.x. PMID 5286110. S2CID 34735617.
External links
| Identifiers: |
|---|
- Carboprost at the US National Library of Medicine Medical Subject Headings (MeSH)