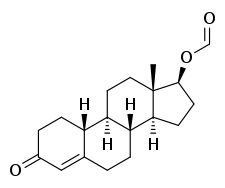
Nandrolone formate
 | |
| Clinical data | |
|---|---|
| Other names | Nandrolone carboxylate; Nandrolone methanoate; 19-Nortestosterone 17β-formate; Estr-4-en-17β-ol-3-one 17β-formate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C19H26O3 |
| Molar mass | 302.414 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Nandrolone formate, also known as nandrolone carboxylate or nandrolone methanoate, as well as 19-nortestosterone 17β-formate or estr-4-en-17β-ol-3-one 17β-formate, is a synthetic, injected anabolic–androgenic steroid (AAS) and a derivative of 19-nortestosterone (nandrolone) that was never marketed.[1][2] It is an androgen ester – specifically, the C17β formate ester of nandrolone.[1][2]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Nandrolone | 20 | 154–155 | <0.1 | 0.5 | 1.6 | 1–16 | 0.1 |
| Testosterone | 1.0–1.2 | 100 | <0.1 | 0.17 | 0.9 | 19–82 | 3–8 |
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Sources: See template. | |||||||
See also
References
- 1 2 Chaudry MA, James KC, Ng CT, Nicholls PJ (1976). "Anabolic and androgenic activities, in rat, of some nandrolone and androstanolone esters". J. Pharm. Pharmacol. 28 (12): 882–5. doi:10.1111/j.2042-7158.1976.tb04085.x. PMID 12263. S2CID 20546783.
- 1 2 Abolghasem Jouyban (26 August 2009). Handbook of Solubility Data for Pharmaceuticals. CRC Press. pp. 125–. ISBN 978-1-4398-0488-9.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.