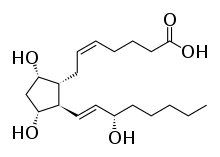
Prostaglandin F2alpha
 | |
| Clinical data | |
|---|---|
| Other names | Amoglandin, Croniben, Cyclosin, Dinifertin, Enzaprost, Glandin, PGF2α, Panacelan, Prostamodin |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous (cannot used to induce labor)because it cannot be used in cervix, intra-amniotic (to induce abortion) |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 3 to 6 hours in amniotic fluid, less than 1 minute in blood plasma |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.209.720 |
| Chemical and physical data | |
| Formula | C20H34O5 |
| Molar mass | 354.487 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 200 mg/mL (20 °C) |
SMILES
| |
InChI
| |
| | |
Prostaglandin F2α (PGF2α in prostanoid nomenclature), pharmaceutically termed carboprost is a naturally occurring prostaglandin used in medicine to induce labor and as an abortifacient.[1] Prostaglandin is also used to treat uterine infections in domestic animals.
In domestic mammals, it is produced by the uterus when stimulated by oxytocin, in the event that there has been no implantation during the luteal phase. It acts on the corpus luteum to cause luteolysis, forming a corpus albicans and stopping the production of progesterone. Action of PGF2α is dependent on the number of receptors on the corpus luteum membrane.
The PGF2α isoform 8-iso-PGF2α was found in significantly increased amounts in patients with endometriosis, thus being a potential causative link in endometriosis-associated oxidative stress.[2]
Mechanism of action
PGF2α acts by binding to the prostaglandin F2α receptor. It is released in response to an increase in oxytocin levels in the uterus, and stimulates both luteolytic activity and the release of oxytocin.[3] Because PGF2α is linked with an increase in uterine oxytocin levels, there is evidence that PGF2α and oxytocin form a positive feedback loop to facilitate the degradation of the corpus luteum.[4] PGF2α and oxytocin also inhibit the production of progesterone, a hormone that facilitates corpus luteum development. Conversely, higher progesterone levels inhibit production of PGF2α and oxytocin, as the effects of the hormones are in opposition to each other.
Pharmaceutical Use
When injected into the body or amniotic sac, PGF2α can either induce labor or cause an abortion depending on the concentration used. In small doses (1–4 mg/day), PGF2α acts to stimulate uterine muscle contractions, which aids in the birth process. However, during the first trimester and in higher concentrations (40 mg/day),[5] PGF2α can cause an abortion by degrading the corpus luteum, which normally acts to maintain pregnancy via the production of progesterone. Since the fetus is not viable outside the womb by this time, the lack of progesterone leads to shedding of the uterine lining and death of the fetus.
Pyometra and uterine infections

Lutalyse is used for the treatment of pyometra in domestic dogs and cats.[6] The drug is also administered to dairy cows in order to reduce uterine infections.[7]
Synthesis
Industrial Synthesis
In 2012 a concise and highly stereoselective total synthesis of PGF2α was described.[8] The synthesis requires only seven steps, a huge improvement on the original 17-steps synthesis of Corey and Cheng,[9] and uses 2,5-dimethoxytetrahydrofuran as a starting reagent, with S-proline as an asymmetric catalyst.
Biosynthesis
In the body PGF2α is synthesized in several distinct steps. First, Phospholipase A2 (PLA2) facilitates the conversion of phospholipids to Arachidonic Acid, the framework from which all prostaglandins are formed.[10] The Arachidonic Acid then reacts with two Cyclooxygenase (COX) receptors, COX-1 and COX-2 to form Prostaglandin H2, an intermediate. Lastly, the compound reacts with Aldose Reductase (AKR1B1) to form PGF2α.[10]
Analogues
The following medications are analogues of prostaglandin F2α:
References
- ↑ The Merck index : an encyclopedia of chemicals, drugs, and biologicals. O'Neil, Maryadele J., Royal Society of Chemistry (Great Britain) (15th ed.). Cambridge, UK: Royal Society of Chemistry. 2013. ISBN 978-1849736701. OCLC 824530529.
{{cite book}}: CS1 maint: others (link) - ↑ Sharma I, Dhaliwal LK, Saha SC, Sangwan S, Dhawan V (June 2010). "Role of 8-iso-prostaglandin F2alpha and 25-hydroxycholesterol in the pathophysiology of endometriosis". Fertility and Sterility. 94 (1): 63–70. doi:10.1016/j.fertnstert.2009.01.141. PMID 19324352.
- ↑ Samuelsson B, Goldyne M, Granström E, Hamberg M, Hammarström S, Malmsten C (1978). "Prostaglandins and thromboxanes". Annual Review of Biochemistry. 47: 997–1029. doi:10.1146/annurev.bi.47.070178.005025. PMID 209733.
- ↑ Hooper SB, Watkins WB, Thorburn GD (December 1986). "Oxytocin, oxytocin-associated neurophysin, and prostaglandin F2 alpha concentrations in the utero-ovarian vein of pregnant and nonpregnant sheep" (PDF). Endocrinology. 119 (6): 2590–7. doi:10.1210/endo-119-6-2590. PMID 3465529.
- ↑ "Dinoprost tromethamine Injection Advanced Patient Information". Truvn Health Analytics Inc. 2016. Retrieved November 2, 2017.
- ↑ Davidson, A P; Nelson, R W; Feldman, E C (1992). "Treatment of pyometra in cats, using prostaglandin F2 alpha: 21 cases (1982-1990)". Journal of the American Veterinary Medical Association. National Library of Medicine. 200 (6): 825–828. PMID 1568932. Retrieved 2 December 2021.
- ↑ MENINO (director), A. "Evaluation of Single Lutalyse Injection Protocol to Reduce Uterine Infections and Improve Reproductive Efficiency in Postpartum Dairy Cows". USDA. OREGON STATE UNIVERSITY. Retrieved 2 December 2021.
- ↑ Coulthard G, Erb W, Aggarwal VK (September 2012). "Stereocontrolled organocatalytic synthesis of prostaglandin PGF2α in seven steps". Nature. 489 (7415): 278–81. Bibcode:2012Natur.489..278C. doi:10.1038/nature11411. PMID 22895192. S2CID 205230275.
- ↑ Corey EJ, Cheng XM (1995). The Logic of Chemical Synthesis. Wiley.
- 1 2 Fortier MA, Krishnaswamy K, Danyod G, Boucher-Kovalik S, Chapdalaine P (August 2008). "A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems". Journal of Physiology and Pharmacology. 59 Suppl 1: 65–89. PMID 18802217.