Adenosine A1 receptor
The adenosine A1 receptor[5] is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand.
Biochemistry
A1 receptors are implicated in sleep promotion by inhibiting wake-promoting cholinergic neurons in the basal forebrain.[6] A1 receptors are also present in smooth muscle throughout the vascular system.[7]
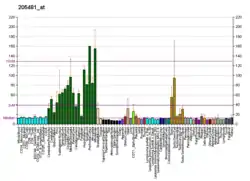
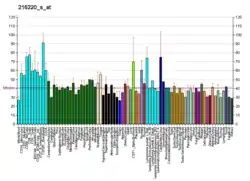
The adenosine A1 receptor has been found to be ubiquitous throughout the entire body.
Signalling
Activation of the adenosine A1 receptor by an agonist causes binding of Gi1/2/3 or Go protein. Binding of Gi1/2/3 causes an inhibition of adenylate cyclase and, therefore, a decrease in the cAMP concentration. An increase of the inositol triphosphate/diacylglycerol concentration is caused by an activation of phospholipase C, whereas the elevated levels of arachidonic acid are mediated by DAG lipase, which cleaves DAG to form arachidonic acid. Several types of potassium channels are activated but N-, P-, and Q-type calcium channels are inhibited.[8]
Mechanism
This receptor has an inhibitory function on most of the tissues in which it rests. In the brain, it slows metabolic activity by a combination of actions. At the neuron's synapse, it reduces synaptic vesicle release.
Ligands
Caffeine, as well as theophylline, has been found to antagonize both A1 and A2A receptors in the brain.
Agonists
- 2-Chloro-N(6)-cyclopentyladenosine (CCPA).
- N6-Cyclopentyladenosine
- N(6)-cyclohexyladenosine
- Tecadenoson is an effective A1 adenosine agonist, as is selodenoson.
- Benzyloxy-cyclopentyladenosine (BnOCPA) is an A1R selective agonist.[9]
PAMs
- 2‑Amino-3-(4′-chlorobenzoyl)-4-substituted-5-arylethynyl thiophene # 4e[10]
Antagonists
- Non-selective
- Selective
- 8-Cyclopentyl-1,3-dimethylxanthine (CPX / 8-cyclopentyltheophylline)
- 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)
- 8-Phenyl-1,3-dipropylxanthine
- Bamifylline
- BG-9719[11]
- BG-9928[12]
- FK-453
- FK-838
- Rolofylline (KW-3902)[13][14]
- N-0861
- ISAM-CV202[15]
In heart
The A1 and A2A receptors of endogenous adenosine are believed to play a role in regulating myocardial oxygen consumption and coronary blood flow. Stimulation of the A1 receptor has a myocardial depressant effect by decreasing the conduction of electrical impulses and suppressing pacemaker cell function, resulting in a decrease in heart rate. This makes adenosine a useful medication for treating and diagnosing tachyarrhythmias, or excessively fast heart rates. This effect on the A1 receptor also explains why there is a brief moment of cardiac standstill when adenosine is administered as a rapid IV push during cardiac resuscitation. The rapid infusion causes a momentary myocardial stunning effect.
In normal physiological states, this serves as protective mechanisms. However, in altered cardiac function, such as hypoperfusion caused by hypotension, heart attack or cardiac arrest caused by nonperfusing bradycardias, adenosine has a negative effect on physiological functioning by preventing necessary compensatory increases in heart rate and blood pressure that attempt to maintain cerebral perfusion.
In neonatal medicine
Adenosine antagonists are widely used in neonatal medicine;
Because a reduction in A1 expression appears to prevent hypoxia-induced ventriculomegaly and loss of white matter, the pharmacological blockade of A1 may have clinical utility.
Theophylline and caffeine are nonselective adenosine antagonists that are used to stimulate respiration in premature infants.
However, we are unaware of clinical studies that have examined the incidence of periventricular leukomalacia (PVL) as related to neonatal caffeine use. Caffeine may reduce cerebral blood flow in premature infants, it is presumed by blocking vascular A2 ARs. Thus, it may prove more advantageous to use selective A1 antagonists to help reduce adenosine-induced brain injury.
References
- GRCh38: Ensembl release 89: ENSG00000163485 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000042429 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Townsend-Nicholson A, Baker E, Schofield PR, Sutherland GR (March 1995). "Localization of the adenosine A1 receptor subtype gene (ADORA1) to chromosome 1q32.1". Genomics. 26 (2): 423–425. doi:10.1016/0888-7543(95)80236-F. PMID 7601478.
- Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, et al. (February 2007). "Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study". The Journal of Neuroscience. 27 (9): 2410–2415. doi:10.1523/JNEUROSCI.5066-06.2007. PMC 6673478. PMID 17329439.
- Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ (March 2005). "Role of A1 adenosine receptors in regulation of vascular tone". American Journal of Physiology. Heart and Circulatory Physiology. 288 (3): H1411–H1416. doi:10.1152/ajpheart.00684.2004. PMID 15539423. S2CID 916788.
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (December 2001). "International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors". Pharmacological Reviews. 53 (4): 527–552. PMC 9389454. PMID 11734617.
- Wall MJ, Hill E, Huckstepp R, Barkan K, Deganutti G, Leuenberger M, et al. (July 2022). "Selective activation of Gαob by an adenosine A1 receptor agonist elicits analgesia without cardiorespiratory depression". Nature Communications. 13 (1): 4150. doi:10.1038/s41467-022-31652-2. PMC 9293909. PMID 35851064.
- Romagnoli R, Baraldi PG, IJzerman AP, Massink A, Cruz-Lopez O, Lopez-Cara LC, et al. (September 2014). "Synthesis and biological evaluation of novel allosteric enhancers of the A1 adenosine receptor based on 2-amino-3-(4'-chlorobenzoyl)-4-substituted-5-arylethynyl thiophene". Journal of Medicinal Chemistry. 57 (18): 7673–7686. doi:10.1021/jm5008853. PMID 25181013.
- Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, et al. (March 2002). "BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy". Circulation. 105 (11): 1348–1353. doi:10.1161/hc1102.105264. PMID 11901047.
- Greenberg B, Thomas I, Banish D, Goldman S, Havranek E, Massie BM, et al. (August 2007). "Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo-controlled, dose-escalation study". Journal of the American College of Cardiology. 50 (7): 600–606. doi:10.1016/j.jacc.2007.03.059. PMID 17692744.
- Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC (October 2007). "The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance". Journal of the American College of Cardiology. 50 (16): 1551–1560. doi:10.1016/j.jacc.2007.07.019. PMID 17936154.
- Cotter G, Dittrich HC, Weatherley BD, Bloomfield DM, O'Connor CM, Metra M, Massie BM (October 2008). "The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment". Journal of Cardiac Failure. 14 (8): 631–640. doi:10.1016/j.cardfail.2008.08.010. PMID 18926433.
- Val C, Rodríguez-García C, Prieto-Díaz R, Crespo A, Azuaje J, Carbajales C, et al. (February 2022). "Optimization of 2-Amino-4,6-diarylpyrimidine-5-carbonitriles as Potent and Selective A1 Antagonists". Journal of Medicinal Chemistry. 65 (3): 2091–2106. doi:10.1021/acs.jmedchem.1c01636. PMC 8842224. PMID 35068155.
External links
- "Adenosine Receptors: A1". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. Archived from the original on 2020-09-20. Retrieved 2007-10-25.
- Adenosine+A1+Receptor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human ADORA1 genome location and ADORA1 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P30542 (Adenosine receptor A1) at the PDBe-KB.