Brilaroxazine
Brilaroxazine (developmental code name RP5063), also known as oxaripiprazole,[1][2] is an investigational atypical antipsychotic which is under development by Reviva Pharmaceuticals for the treatment of neuropsychiatric and inflammatory disorders.[3][5][6][7] As of July 2023, it is in phase III clinical trials for schizophrenia. Reviva Pharmaceuticals also intends to investigate brilaroxazine for the treatment of bipolar disorder, major depressive disorder, attention deficit hyperactivity disorder (ADD/ADHD), psychosis/agitation associated with Alzheimer's disease, Parkinson's disease psychosis, as well as the inflammatory disorders pulmonary arterial hypertension (PAH), idiopathic pulmonary fibrosis (IPF), and psoriasis (topical gel).[3][8] The FDA granted brilaroxazine orphan drug designation for the treatment of PAH and IPF.
 | |
| Clinical data | |
|---|---|
| Other names | RP5063; Oxaripiprazole [1][2] |
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >80% [3] |
| Protein binding | >99% |
| Metabolism | Liver (mostly via CYP3A4 (64%) and CYP2D6 (17%)) [4] |
| Elimination half-life | 55 hours [3] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C22H25Cl2N3O3 |
| Molar mass | 450.36 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Brilaroxazine is a further evolution of third-generation antipsychotics and described as a "dopamine-serotonin system stabilizer" due to its unique actions on dopamine and serotonin neurotransmitter systems compared to other antipsychotics.[9][10][11] Clinical data from phase I and phase II trials suggest that brilaroxazine may have favorable efficacy and a significantly improved side effect profile compared to existing third-generation drugs.[9][12]
Pharmacology
Pharmacodynamics
Brilaroxazine acts as a potent partial agonist of D2, D3, D4 and 5-HT1A receptors, and as an antagonist of 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6 and 5-HT7 receptors.[9][11] Brilaroxazine exhibits high affinity for D2S, D2L, D3, D4.4, 5-HT1A, 5-HT2A, 5-HT2B, 5-HT7 and H1 receptors, and moderate affinity for D1, D5, 5-HT2C, 5-HT3, 5-HT6 and α4β2 nicotinic receptors, the serotonin transporter, and the α1B adrenergic receptor.[9][11] It lacks significant affinity for 5-HT1B, α2 adrenergic, and muscarinic acetylcholine receptors, as well as for the norepinephrine and dopamine transporters.[11]
| Site | Ki (nM) | Action | |
|---|---|---|---|
| D1 | 100 | ND | |
| D2L | 0.45 | Partial agonist | |
| D2S | 0.28 | Partial agonist | |
| D3 | 3.7 | Partial agonist | |
| D4 | 6.0 | Partial agonist | |
| D5 | 200 | ND | |
| 5-HT1A | 1.5 | Partial agonist | |
| 5-HT2A | 2.5 | Weak partial agonist/ Antagonist | |
| 5-HT2B | 0.19 | Antagonist | |
| 5-HT2C | 39 | Antagonist | |
| 5-HT3 | 78 | ND | |
| 5-HT6 | 51 | Antagonist | |
| 5-HT7 | 2.7 | Antagonist | |
| H1 | ND | ND | |
| α4β2 nicotinic | 36.3 | ND | |
| SERTTooltip Serotonin transporter | 107 | ND | |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||
Chemistry
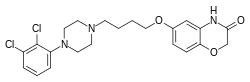
Brilaroxazine is identical to aripiprazole in chemical structure except for the replacement of one of the carbon atoms in aripiprazole's quinolinone ring system with an oxygen atom, resulting instead in a benzoxazinone ring system. This structural change is within the molecule's secondary pharmacophore, which plays a major role in modulating its binding and intrinsic efficacy at dopamine and serotonin receptors.[13][14] The drug is also related structurally to brexpiprazole and cariprazine.
Recent Developments
In August 2023, Reviva Pharmaceuticals announced full enrollment in its RECOVER schizophrenia phase III clinical trial. This pivotal study, which includes clinical sites across the United States, Europe, and Asia, aims to assess the efficacy and longer term safety of brilaroxzaine in schizophrenia. Topline data for the phase 3 study is expected in October 2023. If successful, commercial availability is anticipated in 2026.
References
- Modica MN, Lacivita E, Intagliata S, Salerno L, Romeo G, Pittalà V, Leopoldo M (October 2018). "Structure-Activity Relationships and Therapeutic Potentials of 5-HT7 Receptor Ligands: An Update". J Med Chem. 61 (19): 8475–8503. doi:10.1021/acs.jmedchem.7b01898. hdl:11586/218985. PMID 29767995.
- Jordan, Ann Westcot (14 September 2018). Antidepressants: History, Science, and Issues. Abc-Clio. ISBN 9781440839276.
- "Reviva Corporate Presentation". Reviva Pharmaceuticals. Retrieved 2023-07-19.
- Cantillon M, Ings R, Prakash A, Bhat L (2018). "A Population Pharmacokinetic and Pharmacodynamic Analysis of RP5063 Phase 2 Study Data in Patients with Schizophrenia or Schizoaffective Disorder". European Journal of Drug Metabolism and Pharmacokinetics. 43 (5): 573–585. doi:10.1007/s13318-018-0472-z. PMC 6133081. PMID 29619682.
- Medicines in Development for Mental Health (PDF) (Report). Pharmaceutical Research and Manufacturers of America. 2014. p. 20. Retrieved 2015-05-19.
- Köster LS, Carbon M, Correll CU (December 2014). "Emerging drugs for schizophrenia: an update". Expert Opinion on Emerging Drugs. 19 (4): 511–31. doi:10.1517/14728214.2014.958148. PMID 25234340. S2CID 42729570.
- "Drug Development in Schizophrenia: Summary and Table". Pharmaceutical Medicine. 28 (5): 265–271. 2014. doi:10.1007/s40290-014-0070-6. ISSN 1178-2595. S2CID 8513976.
- "Reviva Product Pipeline". Reviva Pharmaceuticals. Retrieved 2023-07-19.
- Cantillon M, Prakash A, Alexander A, Ings R, Sweitzer D, Bhat L (2017). "Dopamine serotonin stabilizer RP5063: A randomized, double-blind, placebo-controlled multicenter trial of safety and efficacy in exacerbation of schizophrenia or schizoaffective disorder". Schizophrenia Research. 189: 126–133. doi:10.1016/j.schres.2017.01.043. PMID 28215471. S2CID 19499381.
- Rajagopal L, Kwon S, Huang M, Michael E, Bhat L, Cantillon M, Meltzer HY (2017). "RP5063, an atypical antipsychotic drug with a unique pharmacologic profile, improves declarative memory and psychosis in mouse models of schizophrenia". Behavioural Brain Research. 332: 180–199. doi:10.1016/j.bbr.2017.02.036. PMID 28373127. S2CID 13047310.
- Marc Cantillon, M.D., Mike Li, MS, Sarath Kanekal, Ph.D., DABT, RAC, Robert M.J. Ings, Ph.D., Grace Tung, RAC, Laxminarayan Bhat (2013). "Refresh: A Phase 2 RP5063 Efficacy and Safety in Schizophrenia and Schizoaffective Disorder" (PDF). American Society of Clinical Psychopharmacology. Retrieved 2015-05-19.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Cantillon M, Ings R, Bhat L (2018). "Initial Clinical Experience of RP5063 Following Single Doses in Normal Healthy Volunteers and Multiple Doses in Patients with Stable Schizophrenia". Clinical & Translational Science. 11 (4): 387–396. doi:10.1111/cts.12545. PMC 6039200. PMID 29637739.
- Klein Herenbrink C, Verma R, Lim HD, Kopinathan A, Keen A, Shonberg J, Draper-Joyce CJ, Scammells PJ, Christopoulos A, Javitch JA, Capuano B, Shi L, Lane JR (2019). "Molecular Determinants of the Intrinsic Efficacy of the Antipsychotic Aripiprazole". ACS Chemical Biology. 14 (8): 1780–1792. doi:10.1021/acschembio.9b00342. PMC 7365685. PMID 31339684.
- Chen Z, Fan L, Wang H, Yu J, Lu D, Qi J, Nie F, Luo Z, Liu Z, Cheng J, Wang S (2021). "Structure-based design of a novel third-generation antipsychotic drug lead with potential antidepressant properties". Nature Neuroscience. 25 (1): 39–49. doi:10.1038/s41593-021-00971-w. PMID 34887590. S2CID 256840074.
External links
- Reviva Pharmaceuticals
- RECOVER phase III schizophrenia trial - ClinicalTrials.gov
- Brilaroxazine (RP5063) - AdisInsight