Aminolevulinic acid
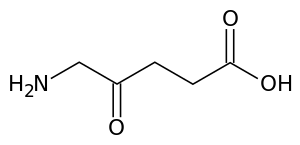 | |
| Names | |
|---|---|
| Trade names | Levulan, NatuALA, Ameluz, others |
| Other names | 5-aminolevulinic acid hydrochloride (5ALA0 |
IUPAC name
| |
| Clinical data | |
| Main uses | Actinic keratoses, basal cell cancer, during surgery for glioma[1][2] |
| Side effects | By mouth: Nausea, fever, low blood pressure, liver problems, diarrhea[2] Topical: Redness, irritation, itchiness, skin peeling[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical, By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607062 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C5H9NO3 |
| Molar mass | 131.131 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 118 °C (244 °F) |
SMILES
| |
InChI
| |
δ-Aminolevulinic acid (dALA, δ-ALA), also known as 5-aminolevulinic acid (5ALA), is a medication used to help visualize cancer tissue during surgery for glioma.[2] It is also used to treat actinic keratoses and basal cell cancer.[1] It is taken by mouth or applied to the skin.[2][1]
Common side effects when taken by mouth include nausea, fever, low blood pressure, liver problems, and diarrhea.[2] Common side effects when applied to the skin include redness, irritation, itchiness, and skin peeling.[1] Safety in pregnancy is unclear.[3] When applied to the skin, it works by making the area sensitive to light, such that when exposed it results in cell death.[1]
δ-Aminolevulinic acid was approved for medical use in the United States in 1999 and Europe in 2011.[2][1] In the United States 2 grams of gel costs about 340 USD as of 2022.[4]
Medical uses
As a precursor of a photosensitizer, 5ALA is also used as an add-on agent for photodynamic therapy.[5] In contrast to larger photosensitizer molecules, it is predicted by computer simulations to be able to penetrate tumor cell membranes.[6]
Cancer diagnosis
Photodynamic detection is the use of photosensitive drugs with a light source of the right wavelength for the detection of cancer, using fluorescence of the drug.[7] 5ALA, or derivatives thereof, can be used to visualize bladder cancer by fluorescence imaging.[7]
Cancer treatment
Aminolevulinic acid is being studied for photodynamic therapy (PDT) in a number of types of cancer.[8] It is not currently a first line treatment for Barrett's esophagus.[9] Its use in brain cancer is currently experimental.[10] It has been studied in a number of gynecological cancers.[11]
Aminolevulinic acid is indicated in adults for visualization of malignant tissue during surgery for malignant glioma (World Health Organization grade III and IV).[12] It is used to visualise tumorous tissue in neurosurgical procedures.[13] Studies since 2006 have shown that the intraoperative use of this guiding method may reduce the tumour residual volume and prolong progression-free survival in people with malignant gliomas.[14][15] The US FDA approved aminolevulinic acid hydrochloride (ALA HCL) for this use in 2017.[16]
Dosage
It is take as a dose of 20 mg/kg 2 to 4 hours before surgery.[2]
Side effects
Side effects may include liver damage and nerve problems.[9] Hyperthermia may also occur.[10] Deaths have also resulted.[9]
Chemistry
It is an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme[17] in mammals, as well as chlorophyll[18] in plants.
Biosynthesis
In non-photosynthetic eukaryotes such as animals, fungi, and protozoa, as well as the class Alphaproteobacteria of bacteria, it is produced by the enzyme ALA synthase, from glycine and succinyl-CoA. This reaction is known as the Shemin pathway, which occurs in mitochondria.[19]
In plants, algae, bacteria (except for the class Alphaproteobacteria) and archaea, it is produced from glutamic acid via glutamyl-tRNA and glutamate-1-semialdehyde. The enzymes involved in this pathway are glutamyl-tRNA synthetase, glutamyl-tRNA reductase, and glutamate-1-semialdehyde 2,1-aminomutase. This pathway is known as the C5 or Beale pathway.[20][21] In most plastid-containing species, glutamyl-tRNA is encoded by a plastid gene, and the transcription, as well as the following steps of C5 pathway, take place in plastids.[22]
Physiology
Activation of mitochondria
In humans, 5ALA is a precursor to heme.[17] Biosynthesized, 5ALA goes through a series of transformations in the cytosol and finally gets converted to Protoporphyrin IX inside the mitochondria.[23][24] This protoporphyrin molecule chelates with iron in presence of enzyme ferrochelatase to produce Heme.[23][24]
Heme increases the mitochondrial activity thereby helping in activation of respiratory system Krebs Cycle and Electron Transport Chain[25] leading to formation of adenosine triphosphate (ATP) for adequate supply of energy to the body.[25]
Accumulation of protoporphyrin IX
Cancer cells lack or have reduced ferrochelatase activity and this results in accumulation of Protoporphyrin IX, a fluorescent substance that can easily be visualized.[7]
Induction of heme oxygenase-1 (HO-1)
Excess heme is converted in macrophages to Biliverdin and ferrous ions by the enzyme HO-1. Biliverdin formed further gets converted to Bilirubin and carbon monoxide.[26] Biliverdin and Bilirubin are potent anti oxidants and regulate important biological processes like inflammation, apoptosis, cell proliferation, fibrosis and angiogenesis.[26]
Plants
In plants, production of 5ALA is the step on which the speed of synthesis of chlorophyll is regulated.[18] Plants that are fed by external 5ALA accumulate toxic amounts of chlorophyll precursor, protochlorophyllide, indicating that the synthesis of this intermediate is not suppressed anywhere downwards in the chain of reaction. Protochlorophyllide is a strong photosensitizer in plants.[27]
References
- 1 2 3 4 5 6 7 "Ameluz". Archived from the original on 20 November 2021. Retrieved 14 January 2022.
- 1 2 3 4 5 6 7 "DailyMed - GLEOLAN- aminolevulinic acid hydrochloride powder, for solution". dailymed.nlm.nih.gov. Archived from the original on 24 March 2021. Retrieved 14 January 2022.
- ↑ "Aminolevulinic acid topical Use During Pregnancy". Drugs.com. Archived from the original on 26 January 2021. Retrieved 14 January 2022.
- ↑ "Ameluz Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 23 April 2021. Retrieved 14 January 2022.
- ↑ Yew, Y.W.; Lai, Y.C.; Lim, Y.L.; Chong, W.S.; Theng, C. (2016). "Photodynamic therapy with topical 5% 5-aminolevulinic acid for the treatment of truncal acne in Asian patients". J Drugs Dermatol. 15 (6): 727–732. PMID 27272080.
- ↑ Erdtman, Edvin (2008). "Modelling the behavior of 5-aminolevulinic acid and its alkyl esters in a lipid bilayer". Chemical Physics Letters. 463 (1–3): 178. Bibcode:2008CPL...463..178E. doi:10.1016/j.cplett.2008.08.021.
- 1 2 3 Wagnières, G.., Jichlinski, P., Lange, N., Kucera, P., Van den Bergh, H. (2014). Detection of Bladder Cancer by Fluorescence Cystoscopy: From Bench to Bedside - the Hexvix Story. Handbook of Photomedicine, 411-426.
- ↑ Inoue, K (February 2017). "5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer". International Journal of Urology. 24 (2): 97–101. doi:10.1111/iju.13291. PMID 28191719.
- 1 2 3 Qumseya, BJ; David, W; Wolfsen, HC (January 2013). "Photodynamic Therapy for Barrett's Esophagus and Esophageal Carcinoma". Clinical Endoscopy. 46 (1): 30–7. doi:10.5946/ce.2013.46.1.30. PMC 3572348. PMID 23423151.
- 1 2 Tetard, MC; Vermandel, M; Mordon, S; Lejeune, JP; Reyns, N (September 2014). "Experimental use of photodynamic therapy in high grade gliomas: a review focused on 5-aminolevulinic acid" (PDF). Photodiagnosis and Photodynamic Therapy. 11 (3): 319–30. doi:10.1016/j.pdpdt.2014.04.004. PMID 24905843. Archived (PDF) from the original on 2020-11-05. Retrieved 2021-08-19.
- ↑ Shishkova, N; Kuznetsova, O; Berezov, T (March 2012). "Photodynamic therapy for gynecological diseases and breast cancer". Cancer Biology & Medicine. 9 (1): 9–17. doi:10.3969/j.issn.2095-3941.2012.01.002. PMC 3643637. PMID 23691448.
- ↑ "Gliolan EPAR". European Medicines Agency (EMA). Archived from the original on 17 January 2021. Retrieved 6 January 2021.
- ↑ Eyüpoglu, Ilker Y.; Buchfelder, Michael; Savaskan, Nic E. (2013). "Surgical resection of malignant gliomas—role in optimizing patient outcome". Nature Reviews Neurology. 9 (3): 141–51. doi:10.1038/nrneurol.2012.279. PMID 23358480. S2CID 20352840.
- ↑ Stummer, W; Pichlmeier, U; Meinel, T; Wiestler, OD; Zanella, F; Reulen, HJ (2006). "Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial". Lancet Oncol. 7 (5): 392–401. doi:10.1016/s1470-2045(06)70665-9. PMID 16648043.
- ↑ Eyüpoglu, Ilker Y.; Hore, Nirjhar; Savaskan, Nic E.; Grummich, Peter; Roessler, Karl; Buchfelder, Michael; Ganslandt, Oliver (2012). ""Berger, Mitch, ed. "Improving the Extent of Malignant Glioma Resection by Dual Intraoperative Visualization Approach". PLOS ONE. 7 (9): e44885. doi:10.1371/journal.pone.0044885. PMC 3458892. PMID 23049761.
- ↑ FDA Approves Fluorescing Agent for Glioma Surgery.June 2017
- 1 2 Gardener, L.C.; Cox, T.M. (1988). "Biosynthesis of heme in immature erythroid cells". The Journal of Biological Chemistry. 263: 6676–6682. doi:10.1016/S0021-9258(18)68695-8.
- 1 2 Wettstein, D.; Gough, S.; Kannangara, C.G. (1995). "Chlorophyll biosynthesis". Plant Cell. 7 (7): 1039–1057. doi:10.1105/tpc.7.7.1039. PMC 160907. PMID 12242396.
- ↑ Ajioka, James; Soldati, Dominique, eds. (September 13, 2007). "22". Toxoplasma: Molecular and Cellular Biology (1 ed.). Taylor & Francis. p. 415. ISBN 9781904933342
- ↑ Beale, SI (1990). "Biosynthesis of the Tetrapyrrole Pigment Precursor, delta-Aminolevulinic Acid, from Glutamate". Plant Physiol. 93 (4): 1273–9. doi:10.1104/pp.93.4.1273. PMC 1062668. PMID 16667613.
- ↑ Willows, R.D. (2004). "Chlorophylls". In Goodman, Robert M. Encyclopaedia of Plant and Crop Science. Marcel Dekker. pp. 258–262. ISBN 0-8247-4268-0
- ↑ Biswal, Basanti; Krupinska, Karin; Biswal, Udaya, eds. (2013). Plastid Development in Leaves during Growth and Senescence (Advances in Photosynthesis and Respiration). Dordrecht: Springer. p. 508. ISBN 9789400757233
- 1 2 Malik, Z; Djaldetti, M (1979). "5 aminolevulinic acid stimulation of porphyrin and hemoglobin synthesis by uninduced Friend erythroleukemic cells". Cell Differentiation. 8 (3): 223–33. doi:10.1016/0045-6039(79)90049-6. PMID 288514.
- 1 2 Olivo, M.; Bhuvaneswari, R.; Keogh, I. (2011). "Advances in Bio-Optical Imaging for the Diagnosis of Early Oral Cancer". Pharmaceutics. 3 (3): 354–378. doi:10.3390/pharmaceutics3030354. PMC 3857071. PMID 24310585.
- 1 2 Ogura S, Maruyama K, Hagiya Y, Sugiyama Y, Tsuchiya K, Takahashi K, Fuminori A, Tabata K, Okura I, Nakajima M, Tanaka T (2011). "The effect of 5-aminolevulinic acid on cytochrome c oxidase activity in liver mouse". BMC Research Notes. 17 (4): 6. doi:10.1186/1756-0500-4-66. PMC 3068109. PMID 21414200.
- 1 2 Loboda, A; Damulewicz, M; Pyza, E; Jozkowicz, A; Dulak, J (2016). "Role of Nrf2/HO-1 system in development, oxidative stress response and disease: an evolutionary conserved mechanism". Cell Mol Life Sci. 73 (17): 3221–47. doi:10.1007/s00018-016-2223-0. PMC 4967105. PMID 27100828.
- ↑ Kotzabasis, K.; Senger, H. (1990). "The influence of 5-aminolevulinic acid on protochlorophyllide and protochlorophyll accumulation in dark-grown Scenedesmus". Z. Naturforsch. 45 (1–2): 71–73. doi:10.1515/znc-1990-1-212. S2CID 42965243.
External links
| Identifiers: |
|---|
- "Aminolevulinic acid". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-10-13. Retrieved 2021-08-19.