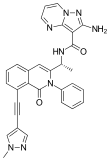
Eganelisib
| |||
| Clinical data | |||
|---|---|---|---|
| Routes of administration | Oral | ||
| ATC code |
| ||
| Identifiers | |||
IUPAC name
| |||
| CAS Number | |||
| PubChem CID | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEMBL | |||
| PDB ligand | |||
| Chemical and physical data | |||
| Formula | C30H24N8O2 | ||
| Molar mass | 528.576 g·mol−1 | ||
| 3D model (JSmol) | |||
SMILES
| |||
InChI
| |||
Eganelisib (USAN), codenamed IPI-549, is an experimental drug being investigated as a possible treatment for cancer. It is a highly selective phosphoinositide 3-kinase inhibitor, and thus works by inhibiting the enzyme PIK3CG, disrupting the PI3K/AKT/mTOR signaling pathway which plays important roles in the development of cancer.[1]
Eganelisib is being developed by Infinity Pharmaceuticals. Early clinical trial results were published in September 2016.[2] On September 29, 2020, it was granted Fast Track designation by the United States Food and Drug Administration (FDA) as a treatment for inoperable, locally advanced, or metastatic triple-negative breast cancer, combined with a checkpoint inhibitor and chemotherapy.[3]
As of October 2020, five phase I/II clinical trials were ongoing in the United States, and one in Europe.[4]
References
- ↑ Evans CA, Liu T, Lescarbeau A, Nair SJ, Grenier L, Pradeilles JA, Glenadel Q, Tibbitts T, Rowley AM, DiNitto JP, Brophy EE, O'Hearn EL, Ali JA, Winkler DG, Goldstein SI, O'Hearn P, Martin CM, Hoyt JG, Soglia JR, Cheung C, Pink MM, Proctor JL, Palombella VJ, Tremblay MR, Castro AC (September 2016). "Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate". ACS Med Chem Lett. 7 (9): 862–7. doi:10.1021/acsmedchemlett.6b00238. PMC 5018865. PMID 27660692.
- ↑ Corey Williams (2016-09-27). "Infinity Pharmaceuticals Inc. Presents Initial Clinical And New Preclinical Data On IPI-549 At Second CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference". The Smarter Analyst. Retrieved 2020-10-30.
- ↑ "Infinity Receives Fast Track Designation for Eganelisib in Combination with a Checkpoint Inhibitor and Chemotherapy for First". Bloomberg (Press release). 2020-09-29. Retrieved 2020-10-30.
- ↑ "CID 91933883 | C30H24N8O2 - PubChem: ClinicalTrials.gov". PubChem. Retrieved 2020-10-30.
Further reading
- Drew SL, Thomas-Tran R, Beatty JW, Fournier J, Lawson KV, Miles DH, Mata G, Sharif EU, Yan X, Mailyan AK, Ginn E, Chen J, Wong K, Soni D, Dhanota P, Chen PY, Shaqfeh SG, Meleza C, Pham AT, Chen A, Zhao X, Banuelos J, Jin L, Schindler U, Walters MJ, Young SW, Walker NP, Leleti MR, Powers JP, Jeffrey JL (October 2020). "Discovery of Potent and Selective PI3Kγ Inhibitors". J Med Chem. 63 (19): 11235–11257. doi:10.1021/acs.jmedchem.0c01203. PMID 32865410.
{{cite journal}}: CS1 maint: uses authors parameter (link)