6'-Guanidinonaltrindole
 | |
| Names | |
|---|---|
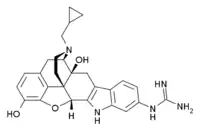
| Preferred IUPAC name
N-[(4bS,8R,8aS,14bR)-7-(Cyclopropylmethyl)-1,8a-dihydroxy-5,6,7,8,8a,9,14,14b-octahydro-4,8-methano[1]benzofuro[2,3-b]pyrido[4,3-c]carbazol-12-yl]guanidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C27H29N5O3 |
| Molar mass | 471.561 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
6′-Guanidinonaltrindole (6′-GNTI) is a κ–δ-opioid receptor selective ligand used in scientific research.[1]
With 6′-GNTI, evidence was provided for the first time that receptor oligomerization plays functional role in living organisms.[2]
6′-GNTI is an extremely biased agonist of the κ-opioid receptor.[1] It is a potent partial agonist of the G protein pathway but does not recruit the β-arrestin pathway.[1] Due to its functional selectivity for the G protein pathway, 6′-GNTI functions as an antagonist of nonbiased KOR agonists on the β-arrestin pathway.[1] It is thought that 6′-GTNI may be able to produce analgesia without dysphoria and with a lower incidence of tolerance.[1]
See also
References
- 1 2 3 4 5 Rives, ML; Rossillo, M; Liu-Chen, LY; Javitch, JA (2012). "6′-Guanidinonaltrindole (6′-GNTI) is a G protein–biased κ-opioid receptor agonist that inhibits arrestin recruitment". J Biol Chem. 287 (32): 27050–4. doi:10.1074/jbc.C112.387332. PMC 3411045. PMID 22736766.
- ↑ Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL (June 2005). "A heterodimer-selective agonist shows in vivo relevance of G protein–coupled receptor dimers". Proc. Natl. Acad. Sci. U.S.A. 102 (25): 9050–5. Bibcode:2005PNAS..102.9050W. doi:10.1073/pnas.0501112102. PMC 1157030. PMID 15932946.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.