Mesalazine
 | |
| Names | |
|---|---|
| Trade names | Asacol, Lialda, Pentasa, others[1] |
| Other names | mesalamine, 5-aminosalicylic acid, 5-ASA, Mesalazine (USAN US) |
IUPAC name
| |
| Clinical data | |
| Drug class | Aminosalicylate[1][2] |
| Main uses | Ulcerative colitis, Crohn's disease[1] |
| Side effects | Headache, nausea, abdominal pain, fever[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | By mouth, rectal |
| Defined daily dose | 1.5 gram[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688021 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | orally: 20–30% absorbed rectally: 10–35% |
| Metabolism | Rapidly & extensively metabolised intestinal mucosal wall and the liver |
| Elimination half-life | 5 hours after initial dose. At steady state 7 hours |
| Chemical and physical data | |
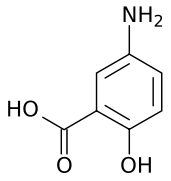
| Formula | C7H7NO3 |
| Molar mass | 153.137 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 283 °C (541 °F) |
SMILES
| |
InChI
| |
Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), is a medication used to treat inflammatory bowel disease, including ulcerative colitis and Crohn's disease.[1] It is generally used for mildly to moderately severe disease.[1] It is taken by mouth or rectally.[1] The formulations which are taken by mouth appear to be similarly effective.[2]
Common side effects include headache, nausea, abdominal pain, and fever.[1] Serious side effects may include pericarditis, liver problems, and kidney problems.[1][2] Use in pregnancy and breastfeeding appears safe.[2] In people with a sulfa allergy certain formulations may result in problems.[1] Mesalazine is an aminosalicylate and anti-inflammatory.[1][2] It works by direct contact with the intestines.[1]
Mesalazine was approved for medical use in the United States in 1987.[1][5] It is on the World Health Organization's List of Essential Medicines as an alternative to sulfasalazine.[6] It is available as a generic medication and sold under many brand names worldwide.[1][7] A month supply in the United Kingdom costs the NHS less than £30 as of 2021.[2] In the United States the wholesale cost of this amount is about 288 USD.[8] In 2017, it was the 246th most commonly prescribed medication in the United States, with more than one million prescriptions.[9][10]
Medical uses
It is used to treat inflammatory bowel disease, including ulcerative colitis and Crohn's disease.[1] It is generally used for mildly to moderately severe disease.[1] It is taken by mouth or rectally.[1] The formulations which are taken by mouth appear to be similarly effective.[2]
Dosage
The defined daily dose is 1.5 grams by mouth or rectally.[4]
Side effects
There are no data on use in pregnant women, but the drug does cross the placenta and is excreted in breast milk. The drug should not be used in children under two,[11] people with kidney disease,[11] or people who are allergic to aspirin.[11]
Side effects are primarily gastrointestinal but may also include headache; GI effects include nausea, diarrhea and abdominal pain. There have been scattered reports of various problems when the oral form is used, including: problems caused by myelosuppression (leukopenia, neutropenia, agranulocytosis, aplastic anaemia, and thrombocytopenia), as well as hair loss, peripheral neuropathy, pancreatitis, liver problems, myocarditis and pericarditis, allergic and fibrotic lung reactions, lupus erythematosus-like reactions and rash (including urticaria), drug fever, interstitial nephritis and nephrotic syndrome, usually reversible on withdrawal. Very rarely, use of mesalazine has been associated with an exacerbation of the symptoms of colitis, Stevens Johnson syndrome and erythema multiforme.[11]
Chemistry
Mesalazine is the active moiety of sulfasalazine, which is metabolized to sulfapyridine and mesalazine.[12] It is also the active component of the prodrug balsalazide along with the inert carrier molecule 4-aminobenzoyl-beta-alanine.[13]
Society and culture
Cost
Several brands are available in the UK and the cost of a month supply varies accordingly.[14] In the United States the wholesale cost of this amount is about 288 USD.[8] In 2017, it was the 246th most commonly prescribed medication in the United States, with more than one million prescriptions.[9][10]
.svg.png.webp) Mesalamine costs (US)
Mesalamine costs (US).svg.png.webp) Mesalamine prescriptions (US)
Mesalamine prescriptions (US)
Brand names
Mesalazine is marketed under various brand names including Apriso, Asacol, Asacol HD, Canasa, Delzicol, Lialda, Pentasa, Rowasa, and Sfrowasa.[15][16]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 "Mesalamine Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 2019-09-04. Retrieved 2019-04-08.
- 1 2 3 4 5 6 7 "1. Gastro-intestinal system". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. pp. 44–47. ISBN 978-0-85711-413-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 "Mesalamine Use During Pregnancy". Drugs.com. 18 September 2018. Archived from the original on 30 December 2019. Retrieved 30 December 2019.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 27 July 2020. Retrieved 8 September 2020.
- ↑ "Asacol HD- mesalamine tablet, delayed release". DailyMed. 15 April 2018. Archived from the original on 16 September 2020. Retrieved 30 December 2019.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "ANDA Approval Reports - 2017 First Generic Drug Approvals". Food and Drug Administration (FDA). Archived from the original on 12 April 2019. Retrieved 8 April 2019.
- 1 2 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Mesalamine - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- 1 2 3 4 "Asacol 400mg MR Tablets - Summary of Product Characteristics (SmPC)". (emc). 14 April 2016. Archived from the original on 3 December 2019. Retrieved 30 December 2019.
- ↑ Richard Finkel R, Clark MA, Cubeddu LX. Lippincott's Illustrated Reviews: Pharmacology, (Fourth ed.). ISBN 978-0-7817-7155-9.
- ↑ "Balsalazide: increasing the choice for patients with ulcerative colitis". Drugs & Therapy Perspectives. 19 (1–4). 2003. doi:10.2165/00042310-200319100-00001.
- ↑ Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. pp. 46–47. ISBN 978-0-7020-7442-4. Archived from the original on 2021-05-22. Retrieved 2021-11-09.
- ↑ "Substance Name: Mesalamine [USAN:USP]". ChemIDplus. Archived from the original on 29 October 2020. Retrieved 2 October 2020.
- ↑ "Mesalamine Uses, Side Effects & Warnings". Drugs.com. 30 August 2019. Archived from the original on 26 January 2021. Retrieved 2 October 2020.
External links
| Identifiers: |
|---|
- "Mesalamine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2019-12-30. Retrieved 2019-12-30.