Nandrolone hexyloxyphenylpropionate
 | |
| Clinical data | |
|---|---|
| Trade names | Anador, Anadur, Anadurine |
| Other names | NHPP; 19-Nortestosterone 17β-(3-(4-hexyloxy)phenyl)propionate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Pharmacokinetic data | |
| Elimination half-life | Intramuscular: 20 days[1][2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.052.538 |
| Chemical and physical data | |
| Formula | C33H46O4 |
| Molar mass | 506.727 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Nandrolone hexyloxyphenylpropionate (NHPP; brand names Anador, Anadur, Anadurine), also known as 19-nortestosterone 17β-(3-(4-hexyloxy)phenyl)propionate, is a synthetic androgen and anabolic steroid and a nandrolone ester that is marketed in France, Denmark, Austria, Luxembourg, and Turkey.[3][4][5][6] It has been studied as a potential long-acting injectable male contraceptive, though it has not been marketed for this indication.[7] Approximately 70% of men became azoospermic, while the remaining men all became oligospermic.[7] NHPP has a mean residence time in the body of 29.1 days and an elimination half-life in the body of 20.1 days.[1]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Nandrolone | 20 | 154–155 | <0.1 | 0.5 | 1.6 | 1–16 | 0.1 |
| Testosterone | 1.0–1.2 | 100 | <0.1 | 0.17 | 0.9 | 19–82 | 3–8 |
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Sources: See template. | |||||||
Hormone levels with nandrolone esters by intramuscular injection
 Nandrolone levels after a single 50, 100, or 150 mg intramuscular injection of nandrolone decanoate in oil solution in men.[8]
Nandrolone levels after a single 50, 100, or 150 mg intramuscular injection of nandrolone decanoate in oil solution in men.[8]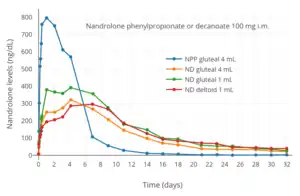 Nandrolone levels after a single 100 mg intramuscular injection of nandrolone decanoate or nandrolone phenylpropionate in 4 mL or 1 mL arachis oil solution into gluteal or deltoid muscle in men.[9]
Nandrolone levels after a single 100 mg intramuscular injection of nandrolone decanoate or nandrolone phenylpropionate in 4 mL or 1 mL arachis oil solution into gluteal or deltoid muscle in men.[9]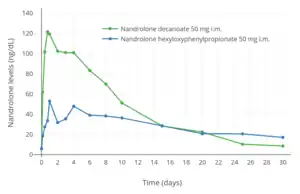 Nandrolone levels with a single 50 mg intramuscular injection of nandrolone decanoate or nandrolone hexyloxyphenylpropionate in oil solution in men.[10]
Nandrolone levels with a single 50 mg intramuscular injection of nandrolone decanoate or nandrolone hexyloxyphenylpropionate in oil solution in men.[10]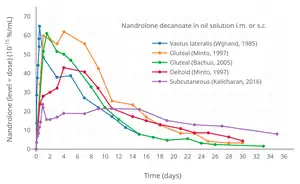 Dose-normalized nandrolone exposure (serum level divided by dose administered) with nandrolone decanoate in oil solution by intramuscular or subcutaneous injection in men.[11][12]
Dose-normalized nandrolone exposure (serum level divided by dose administered) with nandrolone decanoate in oil solution by intramuscular or subcutaneous injection in men.[11][12]
See also
References
- 1 2 Nieschlag E, Behre HM (6 December 2012). Testosterone: Action - Deficiency - Substitution. Springer Science & Business Media. pp. 127–128. ISBN 978-3-662-00814-0.
- ↑ Lin GC, Erinoff L (July 1996). Anabolic Steroid Abuse. DIANE Publishing. pp. 125–. ISBN 978-0-7881-2969-8.
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 660–. ISBN 978-1-4757-2085-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 716–717. ISBN 978-3-88763-075-1.
- ↑ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- ↑ Mozayani A, Raymon L (15 October 2003). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 501–. ISBN 978-1-59259-654-6.
- 1 2 Payne AH, Hardy MP (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 423–. ISBN 978-1-59745-453-7.
- ↑ Bagchus WM, Smeets JM, Verheul HA, De Jager-Van Der Veen SM, Port A, Geurts TB (2005). "Pharmacokinetic evaluation of three different intramuscular doses of nandrolone decanoate: analysis of serum and urine samples in healthy men". J. Clin. Endocrinol. Metab. 90 (5): 2624–30. doi:10.1210/jc.2004-1526. PMID 15713722.
- ↑ Minto CF, Howe C, Wishart S, Conway AJ, Handelsman DJ (1997). "Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and injection volume". J. Pharmacol. Exp. Ther. 281 (1): 93–102. PMID 9103484.
- ↑ Belkien L, Schürmeyer T, Hano R, Gunnarsson PO, Nieschlag E (May 1985). "Pharmacokinetics of 19-nortestosterone esters in normal men". J. Steroid Biochem. 22 (5): 623–9. doi:10.1016/0022-4731(85)90215-8. PMID 4010287.
- ↑ Kalicharan RW, Schot P, Vromans H (February 2016). "Fundamental understanding of drug absorption from a parenteral oil depot". Eur J Pharm Sci. 83: 19–27. doi:10.1016/j.ejps.2015.12.011. PMID 26690043.
- ↑ Kalicharan, Raween Wikesh (2017). New Insights into Drug Absorption from Oil Depots (PhD). Utrecht University.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.