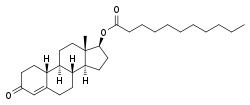
Nandrolone undecanoate
 | |
| Clinical data | |
|---|---|
| Trade names | Dynabolon, Dynabolin, Psychobolan |
| Other names | NU; Nandrolone undecylate; 19-Nortestosterone 17β-undecanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.573 |
| Chemical and physical data | |
| Formula | C29H46O3 |
| Molar mass | 442.684 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Nandrolone undecanoate (NU), also known as nandrolone undecylate, and sold under the brand names Dynabolon, Dynabolin, and Psychobolan, is an androgen and anabolic steroid medication and a nandrolone ester. It was developed in the 1960s, and was previously marketed in France, Germany, Italy, and Monaco, but has since been discontinued and is now no longer known to be available.[1][2][3][4] The pharmacokinetics of nandrolone undecanoate alone (Dynabolon) and in combination with other steroid esters (Trophobolene) have been studied and compared.[5]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Nandrolone | 20 | 154–155 | <0.1 | 0.5 | 1.6 | 1–16 | 0.1 |
| Testosterone | 1.0–1.2 | 100 | <0.1 | 0.17 | 0.9 | 19–82 | 3–8 |
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Sources: See template. | |||||||
See also
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 660–. ISBN 978-1-4757-2085-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 716–717. ISBN 978-3-88763-075-1.
- ↑ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- ↑ Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 477–. ISBN 978-0-9828280-1-4.
- ↑ Courtot, D; Forichon, J; Paris, J (1983). "Pharmacokinetics of 19-Nortestosterone in Man". Chromatography in Biochemistry, Medicine, and Environmental Research, 1: Proceedings of the 1st International Symposium on Chromatography in Biochemistry, Medicine and Environmental Research, Venice, June 16–17, 1981. Analytical Chemistry Symposia Series. Vol. 13. Amsterdam/Oxford/New York: Elsevier. pp. 95–110.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.