Vorinostat
 | |
| Names | |
|---|---|
| Pronunciation | Vorinostat /vɒˈrɪnoʊstæt/ vorr-IN-oh-stat Zolinza (/zoʊˈlɪnzə/ zoh-LIN-zə |
| Trade names | Zolinza, Vorinostat MSD, others |
| Other names | Suberanilohydroxamic acid (SAHA) |
IUPAC name
| |
| Clinical data | |
| Drug class | Histone deacetylase inhibitors (HDI)[1] |
| Main uses | Cutaneous T cell lymphoma (CTCL)[1] |
| Side effects | Diarrhea, tiredness, change in taste, low platelets, hair loss, cough, fever[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (capsules) |
| Typical dose | 400 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607050 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 1.8–11%[2] |
| Protein binding | ~71% |
| Metabolism | Liver glucuronidation and β-oxidation CYP system not involved |
| Metabolites | vorinostat O-glucuronide, 4-anilino-4-oxobutanoic acid (both inactive)[3] |
| Elimination half-life | ~2 hours (vorinostat and O-glucuronide), 11 hours (4-anilino-4-oxobutanoic acid) |
| Excretion | Kidney (negligible) |
| Chemical and physical data | |
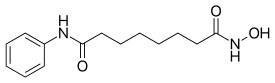
| Formula | C14H20N2O3 |
| Molar mass | 264.325 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vorinostat, sold under the brand name Zolinza, is a medication used for cutaneous T cell lymphoma (CTCL).[1] Specifically it is used when the disease persists, gets worse, or comes back during or after two other treatments.[1] It is taken by mouth.[1]
Common side effects include diarrhea, tiredness, change in taste, low platelets, hair loss, cough, and fever.[1] Other severe side effects may include blood clots and high blood sugar.[1] Use during pregnancy may harm the baby.[1] It is a histone deacetylase inhibitors (HDI).[1]
Vorinostat was approved for medical use in the United States in 2006.[1] While it was given orphan medication status in Europe in 2004, in 2009 the application for approval was withdrawn.[4] In the United States it costs about 14,600 USD per month as of 2021.[5]
Medical uses
Vorinostat is used for CTCL.[6] The European Medicines Agency in 2009 viewed the benefit as being unclear and thus it was not approved for use their.[4]
Dosage
The typical dose is 400 mg taken once per day.[1]
Mechanism of action
Vorinostat has been shown to bind to the active site of histone deacetylases and act as a chelator for zinc ions also found in the active site of histone deacetylases.[7] Vorinostat's inhibition of histone deacetylases results in the accumulation of acetylated histones and acetylated proteins, including transcription factors crucial for the expression of genes needed to induce cell differentiation.[7] It acts on class I, II and IV of histone deacetylase.
History
The compound was developed by Columbia University chemist Ronald Breslow and Memorial Sloan-Kettering researcher Paul Marks.[8][9]
Vorinostat was the first histone deacetylase inhibitor[10] approved by the U.S. Food and Drug Administration (FDA) for the treatment of CTCL on October 6, 2006.[6]
Research
It failed to demonstrate efficacy in treating acute myeloid leukemia in a phase II study.[11]
Vorinostat has also been used to treat Sézary syndrome, another type of lymphoma closely related to CTCL.[12]
A study suggested that vorinostat also possesses some activity against recurrent glioblastoma multiforme, resulting in a median overall survival of 5.7 months (compared to 4–4.4 months in earlier studies).[13] Further brain tumor trials are planned in which vorinostat will be combined with other drugs.
Including vorinostat in treatment of advanced non-small-cell lung carcinoma (NSCLC) showed improved response rates and increased median progression free survival and overall survival.[14]
It has given encouraging results in a phase II trial for myelodysplastic syndromes in combination with idarubicin and cytarabine.[15]
See also
- Trichostatin A
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Vorinostat Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2016. Retrieved 16 September 2021.
- ↑ "Withdrawal Assessment Report for Vorinostat MSD 100 mg Hard Capsules (vorinostat)" (PDF). European Medicines Agency. 23 October 2008. p. 9. Archived (PDF) from the original on 15 September 2016. Retrieved 1 September 2016.
- ↑ "Zolinza (vorinostat) Capsules. Full Prescribing Information" (PDF). Merck & Co., Inc., Whitehouse Station, NJ 08889, USA. Archived (PDF) from the original on 2 October 2016. Retrieved 1 September 2016.
- 1 2 "Vorinostat MSD: Withdrawal of the marketing authorisation application". Archived from the original on 22 September 2021. Retrieved 16 September 2021.
- ↑ "Zolinza Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 14 June 2016. Retrieved 16 September 2021.
- 1 2 "Zolinza (vorinostat) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 21 February 2014. Retrieved 16 February 2014.
- 1 2 Marks PA, Dokmanovic M (December 2005). "Histone deacetylase inhibitors: discovery and development as anticancer agents". Expert Opinion on Investigational Drugs. 14 (12): 1497–511. doi:10.1038/sj.bjc.6603463. PMC 2360770. PMID 16307490.
- ↑ Lee JH, Mahendran A, Yao Y, Ngo L, Venta-Perez G, Choy ML, et al. (September 2013). "Development of a histone deacetylase 6 inhibitor and its biological effects". Proceedings of the National Academy of Sciences of the United States of America. 110 (39): 15704–9. Bibcode:2013PNAS..11015704L. doi:10.1073/pnas.1313893110. PMC 3785767. PMID 24023063.
- ↑ Marks PA, Breslow R (January 2007). "Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug". Nature Biotechnology. 25 (1): 84–90. doi:10.1038/nbt1272. PMID 17211407. Archived from the original on 2021-08-27. Retrieved 2021-07-12.
- ↑ "HDAC Inhibitors Base (vorinostat)". Archived from the original on 2020-07-12. Retrieved 2021-07-12.
- ↑ Schaefer EW, Loaiza-Bonilla A, Juckett M, DiPersio JF, Roy V, Slack J, et al. (October 2009). "A phase 2 study of vorinostat in acute myeloid leukemia". Haematologica. 94 (10): 1375–82. doi:10.3324/haematol.2009.009217. PMC 2754953. PMID 19794082.
- ↑ Cuneo A C. "Mycosis fungoides/Sezary's syndrome". Archived from the original on 2008-02-12. Retrieved 2008-02-15.
- ↑ "Vorinostat shows anti-cancer activity in recurrent gliomas" (Press release). Mayo Clinic. June 3, 2007. Archived from the original on 2007-10-10. Retrieved 2007-06-03.
- ↑ Ramalingam SS, Maitland ML, Frankel P, Argiris AE, Koczywas M, Gitlitz B, et al. (January 2010). "Carboplatin and Paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer". Journal of Clinical Oncology. 28 (1): 56–62. doi:10.1200/JCO.2009.24.9094. PMC 2799233. PMID 19933908.
- ↑ "Zolinza, Idarubicin, Cytarabine Combination Yields High Response Rates In MDS Patients (ASH 2011)". Archived from the original on 2014-10-30. Retrieved 2021-07-12.
External links
| Identifiers: |
|---|
- Vorinostat bound to proteins Archived 2021-08-27 at the Wayback Machine in the PDB