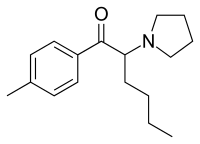
4'-Methyl-α-pyrrolidinohexiophenone
 | |
| Clinical data | |
|---|---|
| Other names | MPHP |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H25NO |
| Molar mass | 259.393 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
4'-Methyl-α-pyrrolidinohexiophenone (MPHP) is a stimulant compound which has been reported as a novel designer drug.[1][2][3] It is closely related to pyrovalerone, being simply its chain-lengthened homologue. In the pyrrolidinophenone series, stimulant activity is maintained so long as the positions of the aryl, ketone and pyrrolidinyl groups are held constant, while the alkyl backbone can be varied anywhere between three and as many as seven carbons,[4] with highest potency usually seen with the pentyl or isohexyl backbone, and a variety of substituents are tolerated on the aromatic ring.[5]
In 2010 a group of researchers from the Institute of Forensic Medicine, University Hospital Jena, Germany concluded that MPHP can lead to serious poisoning with toxic liver damage and rhabdomyolysis.[6]
Legality
In the United States, MPHP is a Schedule I Controlled Substance.[7] Sweden's public health agency suggested to classify MPHP as narcotic on June 1, 2015.[8]
See also
- α-PBP
- α-PHP
- α-PPP
- α-PVP
- MDPHP
- Prolintane
References
- ↑ Springer D, Peters FT, Fritschi G, Maurer HH (June 2003). "New designer drug 4'-methyl-alpha-pyrrolidinohexanophenone: studies on its metabolism and toxicological detection in urine using gas chromatography-mass spectrometry". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 789 (1): 79–91. doi:10.1016/S1570-0232(03)00043-6. PMID 12726846.
- ↑ Peters FT, Dragan CA, Kauffels A, Schwaninger AE, Zapp J, Bureik M, Maurer HH (May 2009). "Biotechnological synthesis of the designer drug metabolite 4'-hydroxymethyl-alpha-pyrrolidinohexanophenone in fission yeast heterologously expressing human cytochrome P450 2D6--a versatile alternative to multistep chemical synthesis". Journal of Analytical Toxicology. 33 (4): 190–7. doi:10.1093/jat/33.4.190. PMID 19470220.
- ↑ Sauer C, Hoffmann K, Schimmel U, Peters FT (May 2011). "Acute poisoning involving the pyrrolidinophenone-type designer drug 4'-methyl-alpha-pyrrolidinohexanophenone (MPHP)". Forensic Science International. 208 (1–3): e20-5. doi:10.1016/j.forsciint.2011.02.026. PMID 21444164.
- ↑ GB patent 1149366, "α-substituted-ketones and processes for their preparation."
- ↑ Meltzer PC, Butler D, Deschamps JR, Madras BK (February 2006). "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors". Journal of Medicinal Chemistry. 49 (4): 1420–32. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278.
- ↑ Sauer C, Hoffmann K, Schimmel U, Peters FT (May 2011). "Acute poisoning involving the pyrrolidinophenone-type designer drug 4'-methyl-alpha-pyrrolidinohexanophenone (MPHP)". Forensic Science International. 208 (1–3): e20-5. doi:10.1016/j.forsciint.2011.02.026. PMID 21444164.
- ↑ "Schedules of Controlled Substances: Temporary Placement of N-Ethylhexedrone, α-PHP, 4-MEAP, MPHP, PV8, and 4-Chloro-α-PVP in Schedule I". Drug Enforcement Administration.
- ↑ "23 nya ämnen kan klassas som narkotika eller hälsofarlig vara". Retrieved 29 June 2015.