6-Methylenedihydrodesoxymorphine
 | |
| Clinical data | |
|---|---|
| Other names | 6-MDDM, 6-Methylene- dihydrodesoxymorphine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H21NO2 |
| Molar mass | 283.371 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
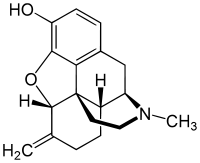
6-Methylenedihydrodesoxymorphine (6-MDDM) is an opiate analogue structurally related to desomorphine that is a derivative of hydromorphone, where the 6-ketone group has been replaced by a methylene group. It has sedative and analgesic effects.
6-Methylenedihydrodesoxymorphine is a potent μ-opioid agonist, 80x stronger than morphine.[1] Compared to morphine it has a faster onset of action and similar duration of effects.[2] It produces around the same degree of respiratory depression as morphine, but less inhibition of gastrointestinal motility. Animal studies show it to be a potent analgesic which produces significant analgesic effects even at low doses while inducing comparatively few side effects,[3] however it has never been developed for medical use in humans.
6-Methylenedihydrodesoxymorphine is synthesised in two steps; first a Wittig reaction is used, reacting hydrocodone with methylenetriphenylphosphorane and an alkyl lithium reagent in diethyl ether to form 6-Methylenedihydrodesoxycodeine. The 3-methoxy group is then cleaved to hydroxy, by reaction with pyridine. The second step tends to be incomplete and often gives fairly low yields, but these can be improved by changing the reaction conditions.[4][5]
See also
References
- ↑ Woster PM. "Chemistry of Opioid Analgesics". PHA 4220 - Neurology Pharmacotherapeutics, Medicinal Chemistry Tutorials. Archived from the original on July 16, 2007.
- ↑ Abdel-Rahman MA, Elliott HW, Binks R, Küng W, Rapoport H (January 1966). "Synthesis and pharmacology of 6-methylenedihydrodesoxymorphine". Journal of Medicinal Chemistry. 9 (1): 1–6. doi:10.1021/jm00319a001. PMID 4163617.
- ↑ Okun R, Elliott HW (November 1958). "Acute pharmacological studies of some new morphine derivatives". The Journal of Pharmacology and Experimental Therapeutics. 124 (3): 255–9. PMID 13588539.
- ↑ Chadha MS, Rapoport H (1957). "The Preparation of Some 6-Methylated Dihydrodesoxymorphines". Journal of the American Chemical Society. 79 (21): 5730–5734. doi:10.1021/ja01578a040.
- ↑ Wiegert PE, De La Mater G, McElheny GC, Patterson LA (1961). "Physical Constants of 6-Methylenedihydrodesoxymorphine". Journal of Organic Chemistry. 26 (12): 5249–5250. doi:10.1021/jo01070a541.