Azidomorphine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Azidomorphine |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.211 |
| Chemical and physical data | |
| Formula | C17H20N4O2 |
| Molar mass | 312.373 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
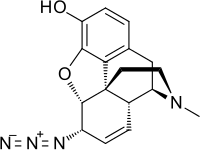
Azidomorphine[1] is an opiate analogue that is a derivative of morphine, where the 7,8 double bond has been saturated and the 6-hydroxy group has been replaced by an azide group.[2]
Azidomorphine binds with high affinity to the mu opioid receptor,[3] and is around 40× more potent than morphine in vivo. It has similar effects to morphine, including analgesia, sedation, and respiratory depression. However, its addiction liability has been found to be slightly lower than that of morphine in animal studies.[4][5]
References
- ↑ GB 1396663, "Analgesic Compositions"
- ↑ Knoll J, Fürst S, Kelemen K (December 1973). "The pharmacology of azidomorphine and azidocodeine". The Journal of Pharmacy and Pharmacology. 25 (12): 929–39. doi:10.1111/j.2042-7158.1973.tb09982.x. PMID 4150295. S2CID 41506653.
- ↑ Horváth K, Wollemann M (November 1986). "Azidomorphine is an agonist of high-affinity opioid receptor binding sites". Neurochemical Research. 11 (11): 1565–9. doi:10.1007/bf00965775. PMID 2825053. S2CID 29580383.
- ↑ Knoll J (1979). "Azidomorphines: a new family of potent analgesics with low dependence capacity". Progress in Neuro-Psychopharmacology. 3 (1–3): 95–108. doi:10.1016/0364-7722(79)90074-2. PMID 45567.
- ↑ Hill RC, Roemer D, Buescher H (June 1977). "Investigations of the analgesic and morphine-like properties of azidomorphine". The Journal of Pharmacology and Experimental Therapeutics. 201 (3): 580–6. PMID 405472.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.