IC-26
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
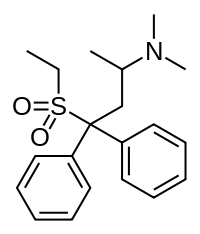
| Formula | C20H27NO2S |
| Molar mass | 345.50 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
IC-26[1] (WIN 1161-3, Methiodone)[2] is an analogue of the opioid analgesic methadone, where the carbonyl group has been replaced by the bioisosteric sulfone group.
Human and animal studies suggest that IC-26 is around the same potency as methadone,[3][4] although other studies have found its activity to be inconsistent between different patients, with consistent opioid activity only being seen at a dose several times that of methadone. IC-26 was assessed for its abuse potential, but despite being found to have similar potential to methadone for development of dependence[5] it was never placed under international control as an illegal drug.
See also
References
- ↑ Tullar BF, Wetterau W, Archer S (November 1948). "The Resolution of Ethyl 1,1-Diphenyl-3-dimethylaminobutyl Sulfone". Journal of the American Chemical Society. 70 (11): 3959–3960. doi:10.1021/ja01191a532. PMID 18207952.
- ↑ US patent 2618640, Archer S, Suter CM, Tullar BF, "Certain amino hydrocarbon sulfones and process of preparation", issued 1952-11-18, assigned to Sterling Drug
- ↑ Lednicer, D. (1982). Central Analgetics. p. 194. ISBN 0-471-08314-3.
- ↑ Janssen PA (1960). "XVIII Sulphones". Diphenylpropylamines. Synthetic Analgesics. Vol. 1. Pergamon Press. pp. 160–163. LCCN 59-13814.
- ↑ Wolbach AB, Fraser HF (1963). "Addiction Liability of I-C-26". Bulletin on Narcotics. UNODC. 1963 (1): 25–28.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.