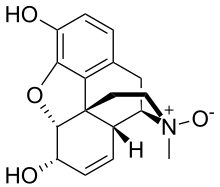
Morphine-N-oxide
 | |
| Names | |
|---|---|
| IUPAC name
(4R,4aR,7S,7aR,12bS)-3-Methyl-2,3,4,4a,7,7a-hexahydro-1H-4,12-methano[1]benzofuro[3,2-e]isoquinoline-7,9-diol 3-oxide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.324 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C17H19NO4 |
| Molar mass | 301.342 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Morphine-N-oxide (genomorphine) is an active opioid metabolite of morphine. Morphine itself, in trials with rats, is 11–22 times more potent than morphine-N-oxide subcutaneously and 39–89 times more potent intraperitoneally. However, pretreatment with amiphenazole or tacrine increases the potency of morphine-N-oxide in relation to morphine (intraperitoneally more so than in subcutaneous administration). A possible explanation is that morphine-N-oxide is rapidly inactivated in the liver and impairment of inactivation processes or enzymes increases functionality.[1]
Morphine-N-oxide can also form as a decomposition product of morphine outside the body and may show up in assays of opium and poppy straw concentrate. Codeine and the semi-synthetics such as heroin, dihydrocodeine, dihydromorphine, hydromorphone, and hydrocodone also have equivalent amine oxide derivatives.
Morphine-N-Oxide has a DEA ACSCN of 9307 and annual production quota of 655 grams in 2013. It is a Schedule I controlled substance in the US.[2]
See also
- Codeine-N-oxide
- Morphine-6-glucuronide
- Morphine-3-glucuronide
References
- ↑ Fennessy, M. R. (1968). "The analgesic action of morphine-n-oxide". British Journal of Pharmacology. 34 (2): 337–344. doi:10.1111/j.1476-5381.1968.tb07055.x. PMC 1703337. PMID 5687589.
- ↑ http://www.deadiversion.usdoj.gov/fed_regs/quotas/2013/fr0620.htm