Pharmacology of bicalutamide
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth[1] |
| Drug class | Nonsteroidal antiandrogen |
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed; absolute bioavailability unknown[2] |
| Protein binding | Racemate: 96.1%[1] (R)-Isomer: 99.6%[1] (Mainly to albumin)[1] |
| Metabolism | Liver (extensively):[3][4] • Hydroxylation (CYP3A4) • Glucuronidation (UGT1A9) |
| Metabolites | • Bicalutamide glucuronide • Hydroxybicalutamide • Hydroxybicalutamide gluc. (All inactive)[3][1][5][6] |
| Elimination half-life | Single-dose: 6 days[7] Continuous: 7–10 days[8] |
| Excretion | Feces: 43%[3] Urine: 34%[3] |
The pharmacology of bicalutamide, a nonsteroidal antiandrogen (NSAA), has been well-characterized. In terms of pharmacodynamics, bicalutamide acts as a selective antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has no capacity to activate the AR. It does not decrease androgen levels and has no other important hormonal activity. The medication has progonadotropic effects due to its AR antagonist activity and can increase androgen, estrogen, and neurosteroid production and levels. This results in a variety of differences of bicalutamide monotherapy compared to surgical and medical castration, such as indirect estrogenic effects and associated benefits like preservation of sexual function and drawbacks like gynecomastia. Bicalutamide can paradoxically stimulate late-stage prostate cancer due to accumulated mutations in the cancer. When used as a monotherapy, bicalutamide can induce breast development in males due to its estrogenic effects. Unlike other kinds of antiandrogens, it may have less adverse effect on the testes and fertility.
In terms of pharmacokinetics, bicalutamide is well-absorbed when taken by mouth. However, absorption diminishes at higher dosages. It reaches maximal constant levels after 4 to 12 weeks of therapy. Bicalutamide shows extensive plasma protein binding, mainly to albumin. It crosses the blood–brain barrier and exerts effects in the central nervous system. Bicalutamide is metabolized in the liver by hydroxylation and glucuronidation. The metabolites of bicalutamide are not known to be active. The medication has a very long biological half-life of 6 days with a single dose and 7 to 10 days with repeated administration. Bicalutamide and its metabolites are eliminated in urine, feces, and bile, mainly in the form of conjugates. The pharmacokinetics of bicalutamide are not influenced by food, age, body weight, renal impairment, or mild-to-moderate hepatic impairment, but ethnicity may influence its pharmacokinetics in some cases.
Pharmacodynamics
| Antiandrogen | AR | PR-B | |||
|---|---|---|---|---|---|
| Ki (nM) | IC50 (nM) | Imax (%) | IC50 (nM) | Imax (%) | |
| Bicalutamide | 117 | 157 | 78 | 1,819 | 88 |
| Cyproterone acetate | 14 | 26 | 48 | >10,000 | 12 |
| Hydroxyflutamide | 27 | 15 | 83 | 2,013 | 90 |
| Mifepristone | 22 | 5 | 75 | 0.18 | 96 |
| Notes: IC50 values are for functional antagonism. Imax is maximal inhibition. Source:[9] | |||||
| Compound | AR | PR | ER | GR | MR |
|---|---|---|---|---|---|
| Bicalutamide | 14–54 | 3,500–7,200 | >1,000,000 | 44,000–320,000 | ≥360,000 |
| Dihydrotestosterone | 0.5–3.1 | 280–440 | 38,000–340,000 | 2,700–20,000 | 2,100–2,300 |
| Notes: Values are Ki or IC50 (nM) for binding inhibition (affinity). Sources:[10][11][12][13] | |||||
Antiandrogenic activity
| Compound | RBA[lower-alpha 2] |
|---|---|
| Metribolone | 100 |
| Dihydrotestosterone | 85 |
| Cyproterone acetate | 7.8 |
| Bicalutamide | 1.4 |
| Nilutamide | 0.9 |
| Hydroxyflutamide | 0.57 |
| Flutamide | <0.0057 |
Notes:
| |
| Antiandrogen | Relative potency |
|---|---|
| Bicalutamide | 4.3 |
| Hydroxyflutamide | 3.5 |
| Flutamide | 3.3 |
| Cyproterone acetate | 1.0 |
| Zanoterone | 0.4 |
| Description: Relative potencies of orally administered antiandrogens in antagonizing 0.8 to 1.0 mg/kg s.c. testosterone propionate-induced ventral prostate weight increase in castrated immature male rats. Sources: See template. | |
Bicalutamide acts as a highly selective competitive silent antagonist of the androgen receptor (AR) (IC50 = 159–243 nM), the major biological target of the androgen sex hormones testosterone and dihydrotestosterone (DHT).[16][17][18][19] It has no capacity to activate the AR under normal physiological circumstances.[20] In addition to competitive antagonism of the AR, bicalutamide has been found to accelerate the degradation of the AR, and this action may also be involved in its activity as an antiandrogen.[21] The activity of bicalutamide lies in the (R)-isomer, which binds to the AR with an affinity that is about 30-fold higher than that of the (S)-isomer.[22] Levels of the (R)-isomer also notably are 100-fold higher than those of the (S)-isomer at steady-state.[23][24]
In relation to its selectivity for the AR, unlike steroidal antiandrogens (SAAs) such as cyproterone acetate (CPA) and megestrol acetate, bicalutamide does not interact importantly with other steroid hormone receptors (including the ERs, PRs, GR, or MR), and in accordance, has no clinically relevant additional, off-target hormonal activity (estrogenic or antiestrogenic, progestogenic or antiprogestogenic, glucocorticoid or antiglucocorticoid, or mineralocorticoid or antimineralocorticoid).[25][26][22][27] However, it has been reported that bicalutamide has weak affinity for the progesterone receptor (PR) (~100- to 500-fold lower than for the AR),[11][12][13][9] where it acts as an antagonist (with only ~12-fold lower functional inhibition relative to the AR in one study).[9][28] Hence, bicalutamide may have some antiprogestogenic activity, although the clinical relevance of this is unknown.[9][28] Bicalutamide does not inhibit 5α-reductase and is not known to inhibit other enzymes involved in androgen steroidogenesis (e.g., CYP17A1).[20] Although bicalutamide does not bind to the ERs, it can increase estrogen levels secondarily to blockade of the AR when used as a monotherapy in males, and for this reason, the medication can indirectly activate the ERs to a degree and hence have some indirect estrogenic effects in men.[29] Also in contrast to SAAs, bicalutamide neither inhibits nor suppresses androgen production in the body (i.e., it does not act as an antigonadotropin or steroidogenesis inhibitor),[2] and instead exclusively mediates its antiandrogen effects by blocking androgen binding and subsequent receptor activation at the level of the AR.[25][22]
In addition to the classical nuclear AR, bicalutamide has also been identified as a potent antagonist of ZIP9, a membrane androgen receptor (mAR) and zinc transporter protein, with an IC50 of 66.3 nM (relative to Kd = 17.9 nM for testosterone).[30] This protein appears to be involved in prostate cancer and breast cancer.[31] Bicalutamide failed to affect testosterone signaling mediated by GPRC6A, another mAR, on the other hand.[32]
| Species | IC50 (nM) | RBA (ratio) | ||||
|---|---|---|---|---|---|---|
| Bicalutamide | 2-Hydroxyflutamide | Nilutamide | Bica / 2-OH-flu | Bica / nilu | Ref | |
| Rat | 190 | 700 | ND | 4.0 | ND | [33] |
| Rat | ~400 | ~900 | ~900 | 2.3 | 2.3 | [34] |
| Rat | ND | ND | ND | 3.3 | ND | [35] |
| Rata | 3595 | 4565 | 18620 | 1.3 | 5.2 | [36] |
| Human | ~300 | ~700 | ~500 | 2.5 | 1.6 | [15] |
| Human | ~100 | ~300 | ND | ~3.0 | ND | [37] |
| Humana | 2490 | 2345 | 5300 | 1.0 | 2.1 | [36] |
| Footnotes: a = Controversial data. Sources: See template. | ||||||
Drug levels, androgen levels, and efficacy
The affinity of bicalutamide for the AR is approximately 30 to 100 times lower than that of DHT (IC50 ≈ 3.8 nM), the main endogenous ligand of the receptor in the prostate gland.[38][19][1][39] However, sufficiently high relative concentrations of bicalutamide (1,000- to 10,000-fold excess) are able to completely prevent activation of the AR by androgens like DHT and testosterone and subsequent upregulation of the transcription of androgen-responsive genes and associated effects.[40][26][41][42] At steady-state, relative to the normal adult male range for testosterone levels (300–1,000 ng/dL),[43] circulating concentrations of bicalutamide at 50 mg/day are roughly 600 to 2,500 times higher and at 150 mg/day around 1,500 to 8,000 times higher than circulating testosterone levels, while bicalutamide concentrations, relative to the mean testosterone levels present in men who have been surgically castrated (15 ng/dL),[44] are approximately 42,000 times higher than testosterone levels at 50 mg/day.[45][46][47][48][49]
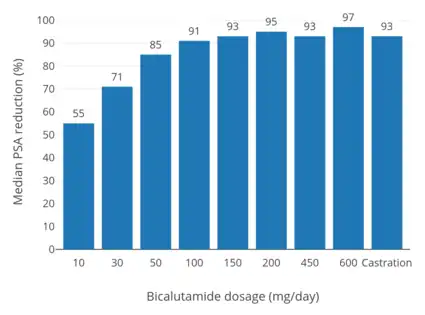
Whereas testosterone is the major circulating androgen, DHT is the major androgen in the prostate gland.[51] DHT levels in circulation are relatively low and only approximately 10% of those of circulating testosterone levels.[51] Conversely, local concentrations of DHT in the prostate gland are 8- to 10-fold higher than circulating levels of DHT.[52][25] This is due to high expression of 5α-reductase in the prostate gland, which very efficiently catalyzes the formation of DHT from testosterone[52] such that over 90% of intraprostatic testosterone is converted into DHT.[53][54] Relative to testosterone, DHT is 2.5- to 10-fold as potent as an AR agonist in bioassays, and hence, is a much stronger androgen in comparison.[55] As such, AR signaling is exceptionally high in the prostate gland, and the effectiveness of bicalutamide monotherapy in the treatment of prostate cancer, which is roughly equivalent to that of gonadotropin-releasing hormone analogues (GnRH analogues),[56][42][57][58] demonstrates a capacity of bicalutamide to strongly antagonize the AR. On the other hand, GnRH analogue monotherapy achieves only a 50 to 60% reduction in levels of DHT in the prostate gland,[19][59] and combined androgen blockade (CAB), the combination of surgical castration or a GnRH analogue and bicalutamide, is significantly more effective than either modality alone in the treatment of prostate cancer.[16][60] Bicalutamide monotherapy has been found to decrease circulating levels of prostate-specific antigen (PSA), a marker of prostate cancer growth, by 57% at 10 mg/day, 73% at 30 mg/day, 90% at 50 mg/day, 97% at 100 mg/day, and 97% at 150 mg/day, while a 97% reduction in PSA is observed with 50 mg/day bicalutamide as a part of CAB.[19] It has also been reported that bicalutamide monotherapy decreases median circulating levels of PSA at 3 months by 86.7% at 100 mg/day, 91.1% at 150 mg/day, and 93.8% at 200 mg/day.[50] Above a bicalutamide monotherapy dosage of 200 mg/day, up to 600 mg/day, decreases in PSA levels reach a plateau.[28]
Despite the high medication levels that are achieved, due to their relatively low affinities for the AR, it has been suggested that 5 to 10% of DHT may remain unblocked in the prostate gland with CAB using standard doses of first-generation NSAAs.[61][62][63] In accordance, second-generation NSAAs like enzalutamide and apalutamide, which have 5- to 10-fold higher affinity for the AR than bicalutamide, have been found to be more effective than bicalutamide in the treatment of prostate cancer.[62] However, in the TERRAIN and STRIVE trials, which compared bicalutamide and enzalutamide as a component of CAB and found that enzalutamide extended life by 3 to 4 times as much time as bicalutamide, the dosage of enzalutamide used (160 mg) was over 3 times that of the dosage of bicalutamide used (50 mg).[64][65] As a result, it has been suggested that the 50 mg/day dosage of bicalutamide used in this study and in CAB in general may be suboptimal.[28][65] This is in accordance with clinical findings that PSA decreases with CAB using bicalutamide plateau at a dosage of bicalutamide of 150 to 200 mg/day.[28]
In women, total testosterone levels are 20-fold and free testosterone levels 40-fold lower relative to men.[66] In addition, whereas bicalutamide monotherapy can increase testosterone levels by up to 2-fold in men,[58][67] the medication does not increase testosterone levels in women.[68][69][70] For these reasons, much lower dosages of bicalutamide (e.g., 25 mg/day in the hirsutism studies) may be used in women with significant antiandrogenic effectiveness.[71][72][73][74]
Influences on hormone levels
| Dosage | Before | Aftera | Difference | Change |
|---|---|---|---|---|
| 10 mg/day | 400 ng/dL | 490–520 ng/dL | +90–120 ng/dL | +21–29% |
| 30 mg/day | 320 ng/dL | 490–550 ng/dL | +170–230 ng/dL | +55–73% |
| 50 mg/day | 370 ng/dL | 550–610 ng/dL | +180–240 ng/dL | +46–62% |
| 100 mg/day | 320 ng/dL | 460–490 ng/dL | +140–170 ng/dL | +45–55% |
| 150 mg/day | 290 ng/dL | 460–490 ng/dL | +170–200 ng/dL | +60–70% |
| 200 mg/day | 320 ng/dL | 520–550 ng/dL | +200–230 ng/dL | +64–73% |
| Footnotes: a = After 29 to 85 days of treatment. Sources: [76] | ||||
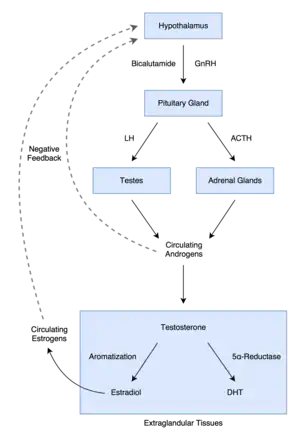
In men, blockade of the AR by bicalutamide in the pituitary gland and hypothalamus prevents the negative feedback of androgens on the hypothalamic–pituitary–gonadal (HPG) axis, resulting in an increase in luteinizing hormone (LH) secretion and levels.[77] Follicle-stimulating hormone (FSH) levels, in contrast, remain essentially unchanged.[78] The increase in LH levels leads to an elevation in androgen and estrogen levels.[79] At a dosage of 150 mg/day, bicalutamide has been found to increase testosterone levels by about 1.5- to 2-fold (59–97% increase) and estradiol levels by about 1.5- to 2.5-fold (65–146% increase).[58][67][74] Levels of DHT are also increased to a lesser extent (by 24–30%), and concentrations of sex hormone-binding globulin (SHBG) and prolactin increase as well (by 8–42% and 40–65%, respectively) secondary to the increase in estradiol levels.[74][80][81] The estradiol concentrations produced in men by bicalutamide monotherapy are said to approximate the low-normal estradiol levels of a premenopausal woman,[67] while testosterone levels generally remain in the high end of the normal male range and rarely exceed it.[82][25] Dosages of bicalutamide of 10 mg, 30 mg, and 50 mg per day have been found to produce a "moderate" effect on sex hormone levels in men with prostate cancer (notably providing indication that the drug has clinically-relevant antiandrogen effects in males at a dosage as low as 10 mg/day).[83][76] The elevated levels of gonadotropins and gonadal steroids associated with NSAA monotherapy is a unique endocrine state which can be described as "hypergonadotropic hypergonadism".[84][85]
Bicalutamide increases androgen and estrogen levels only in men, and does not do so in women.[68][69][70] This is because androgen levels are comparatively far lower in women and in turn exert little to no basal suppression of the HPG axis.[70] Minimal or no changes of importance in levels of total testosterone, free testosterone, dihydrotestosterone, estradiol, androstenedione (A4), dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), 3α-androstanediol glucuronide (3α-ADG), progesterone, 17α-hydroxyprogesterone (17α-OHP), LH, FSH, prolactin, or SHBG have been observed in women with hirsutism with or without polycystic ovary syndrome that were treated with 25 or 50 mg/day bicalutamide for 6 to 12 months.[86][87] However, in one study in women with polycystic ovary syndrome, 25 mg/day bicalutamide significantly decreased levels of total and free testosterone and significantly increased levels of SHBG.[88] In addition to the minimal changes in hormone levels in women, although bicalutamide monotherapy increases gonadotropin and sex hormone levels in men, this will not occur if bicalutamide is combined with an antigonadotropin such as a GnRH analogue, estrogen, or progestogen, as these medications maintain negative feedback on the HPG axis.[20][89][90][91]
The reason that testosterone levels are elevated but almost always remain in the normal male range with bicalutamide monotherapy is thought to be due to the concomitantly increased levels of estradiol, as estradiol is potently antigonadotropic and limits secretion of LH.[77] In fact, estradiol is a much stronger inhibitor of gonadotropin secretion than is testosterone, and even though circulating concentrations of estradiol are far lower than those of testosterone in men, it is said that estradiol is nonetheless likely the major feedback regulator of gonadotropin secretion in this sex.[92] In accordance, clomifene, a selective estrogen receptor modulator with antiestrogenic activity, has been found to increase testosterone levels to as much as 250% of initial values in men with hypogonadism,[93] and a study of clomifene treatment in normal men observed increases in FSH and LH levels of 70–360% and 200–700%, respectively, with increases in testosterone levels that were similar to the increases seen with the gonadotropins.[94][95] In addition to systemic or circulating estradiol, local aromatization of testosterone into estradiol in the hypothalamus and pituitary gland may contribute to suppression of gonadotropin secretion.[92]
Bicalutamide more than blocks the effects of the increased testosterone levels that it induces in men, which is evidenced by the fact that monotherapy with the drug is about as effective as GnRH analogue therapy in the treatment of prostate cancer.[56] However, in contrast, the effects of the elevated estrogen levels remain unopposed by bicalutamide, and this is largely responsible for the feminizing side effects (e.g., gynecomastia) of the drug in men.[96]
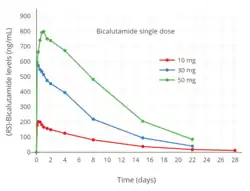
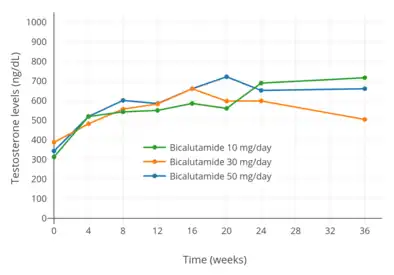 Testosterone levels with 10, 30, and 50 mg/day bicalutamide monotherapy in men with prostate cancer.[97]
Testosterone levels with 10, 30, and 50 mg/day bicalutamide monotherapy in men with prostate cancer.[97]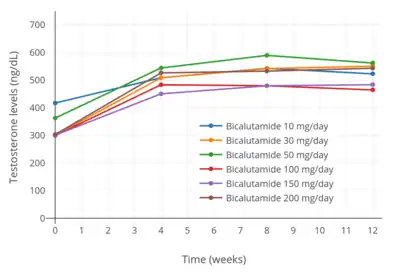 Testosterone levels with 10 to 200 mg/day bicalutamide monotherapy in men with prostate cancer.[76]
Testosterone levels with 10 to 200 mg/day bicalutamide monotherapy in men with prostate cancer.[76]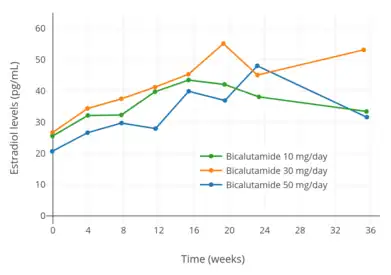 Estradiol levels with 10, 30, and 50 mg/day bicalutamide monotherapy in men with prostate cancer.[97]
Estradiol levels with 10, 30, and 50 mg/day bicalutamide monotherapy in men with prostate cancer.[97]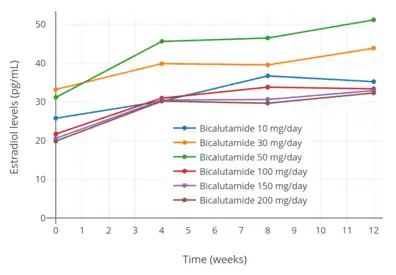 Estradiol levels with 10 to 200 mg/day bicalutamide monotherapy in men with prostate cancer.[76]
Estradiol levels with 10 to 200 mg/day bicalutamide monotherapy in men with prostate cancer.[76]
Differences from castration
It has been proposed that the increase in estrogen levels caused by NSAAs like bicalutamide compensates for androgen blockade in the brain, which may explain differences in the side effect profiles of these drugs relative to GnRH analogues/castration, combined androgen blockade, and CPA (which, in contrast, decrease both androgen and estrogen levels).[98][99][100] In the case of sexual interest and function, this notion is supported by a variety of findings including animal studies showing that estrogen deficiency results in diminished sexual behavior, treatment with tamoxifen resulting in significantly lowered libido in 30% of men receiving it for male breast cancer, and estrogen administration restoring libido and the frequency of sexual intercourse in men with congenital estrogen deficiency, among others.[98][99][100][101]
Several metabolites of testosterone and DHT, including estradiol, 3α-androstanediol, and 3β-androstanediol, are estrogens (mainly potent ERβ agonists in the cases of the latter two), and 3α-androstanediol is additionally a potent GABAA receptor-potentiating neurosteroid.[102][103] Due to the fact that bicalutamide does not lower testosterone levels, the levels of these metabolites would not be expected to be lowered either, unlike with therapies such as GnRH analogues. (Indeed, testosterone, DHT, and estradiol levels are actually raised by bicalutamide treatment, and for this reason, levels of 3α- and 3β-androstanediol might be elevated to some degree similarly.) These metabolites of testosterone have been found to have AR-independent positive effects on sexual motivation,[102][103][104][105] and may explain the preservation of sexual interest and function by bicalutamide and other NSAAs.[98] They also have antidepressant, anxiolytic, and cognitive-enhancing effects,[105][106][107][108] and may account for the lower incidence of depression with bicalutamide and other NSAAs relative to other antiandrogen therapies.
A study found that a combination of bicalutamide and dutasteride, a 5α-reductase inhibitor and inhibitor of neurosteroid biosynthesis, produced fewer sexual side effects than GnRH analogue therapy, specifically suggesting the role of estrogens in the preservation sexual interest and function with bicalutamide monotherapy.[109]
It has been reported that bicalutamide, surprisingly, has no antianabolic effect on testosterone propionate-induced increases in levator ani muscle weight at doses that inhibit and even completely prevent testosterone propionate-induced growth of the prostate gland and seminal vesicles in rats.[110][111] As such, it has been said that, based on preclinical research, bicalutamide does not have marked antianabolic effects.[110] However, higher doses of bicalutamide are able to considerably inhibit the growth of the levator ani muscle in rats.[112] In any case, analogously to animal findings, bicalutamide monotherapy has been found to significantly preserve lean muscle mass and muscle strength in aged men with prostate cancer relative to GnRH agonists.[81] It is notable that in contrast to castration, bicalutamide preserves and increases estrogen levels, and estrogens are thought to have positive effects on skeletal muscle, including on muscle mass.[113]
Paradoxical stimulation of late-stage prostate cancer
Though a pure, or silent antagonist of the AR under normal circumstances, bicalutamide, as well as other earlier antiandrogens like flutamide and nilutamide, have been found to possess weak partial agonist properties in the setting of AR overexpression and agonist activity in the case of certain mutations in the ligand-binding domain (LBD) of the AR.[114][115] As both of these circumstances can eventually occur in prostate cancer, resistance to bicalutamide usually develops and the drug has the potential to paradoxically stimulate tumor growth when this happens.[114][116] This is the mechanism of the phenomenon of antiandrogen withdrawal syndrome, where antiandrogen discontinuation paradoxically slows the rate of tumor growth.[116] The newer drug enzalutamide has been shown not to have agonistic properties in the context of overexpression of the AR, though certain mutations in the AR can still convert it from an antagonist to agonist.[114]
Induction of breast development
| Study | N | Dosage | Gynecomastia | Breast tenderness | Ref |
|---|---|---|---|---|---|
| Tyrrell et al. (1998)a | 386 | 10 mg/day | 9% | 11% | [76] |
| 30 mg/day | 26% | 42% | |||
| 50 mg/day | 36% | 48% | |||
| 100 mg/day | 79% | 86% | |||
| 150 mg/day | 78% | 89% | |||
| 200 mg/day | 79% | 79% | |||
| Kennealey & Furr (1991)b | 210 | 10 mg/day | 29% | 38% | [97] |
| 30 mg/day | 60% | 64% | |||
| 50 mg/day | 52% | 60% | |||
| Zanardi et al. (2006)c | 66 | 0 mg/week (controls) | 0% | 0% | [117][118][119] |
| 50 mg/week (~7 mg/day) | 44% | 32% | |||
| 100 mg/week (~14 mg/day) | 50% | 64% | |||
| Footnotes: a = Testosterone levels increased to ~460–610 ng/dL and estradiol levels to ~32–51 pg/mL. b = Testosterone levels increased to ~505–715 ng/dL and estradiol levels to ~32–53 pg/mL. c = Testosterone levels increased to ~540–600 ng/dL and estradiol levels to ~29–34 pg/mL. | |||||
In transgender women, breast development is a desired effect of antiandrogen and/or estrogen treatment.[120][121] Bicalutamide induces breast development in individuals assigned male at birth by two mechanisms: 1) blocking androgen signaling in breast tissue; and 2) increasing estrogen levels.[122][123] Estrogen is responsible for the induction of breast development under normal circumstances, while androgens powerfully suppress estrogen-induced breast growth.[124][125] It has been found that very low levels of estrogen can induce breast development in the presence of low or no androgen signaling.[124][126][127] In accordance, not only does bicalutamide induce gynecomastia at a high rate when given as a monotherapy to men with prostate cancer (47–85%; 66% in one very large trial),[123] similarly to high-dose estrogen therapy with diethylstilbestrol (41–77%), NSAAs have been found to result in a higher incidence of gynecomastia in combination with a GnRH analogue (13–25%) relative to GnRH analogue therapy or castration alone (1–16%) (in spite of the presence of only castrate levels of estrogen in both cases).[128][29][129][50] The rate of gynecomastia with CAB is also higher than with CPA monotherapy (7%).[128]
A study of men treated with NSAA (flutamide or bicalutamide) monotherapy for prostate cancer found that NSAAs induced full ductal development and moderate lobuloalveolar development of the breasts from a histological standpoint.[130][131][132] The study also found that, in contrast, treatment of transgender women with estrogen and CPA (which is progestogenic in addition to antiandrogenic, unlike NSAAs) resulted in full lobuloalevolar development, as well as pregnancy-like breast hyperplasia in two of the subjects.[130][132] In addition, it was observed that the lobuloalveolar maturation reversed upon discontinuation of CPA after sex reassignment surgery in these individuals.[130] It was concluded that progestogen in addition to antiandrogen/estrogen treatment is required for the induction of full female histological breast development (i.e., that includes complete lobuloalveolar maturation), and that continued progestogen treatment is necessary to maintain such maturation.[130][131] It should be noted however that although these findings may have important implications in the contexts of lactation and breastfeeding, epithelial tissue accounts for approximately only 10% of breast volume (with the bulk of the breasts (80–90%) being represented by stromal or adipose tissue),[133][134][135][136] and it is uncertain to what extent, if any, that development of lobuloalveolar structures (a form of epithelial tissue) contributes to breast size and/or shape.[120]
Effects on spermatogenesis and fertility
Spermatogenesis and male fertility are dependent on FSH, LH, and high levels of testosterone within the testicles.[52][137] LH does not seem to be involved in spermatogenesis outside of its role in inducing production of testosterone by the Leydig cells in the seminiferous tubules (which make up approximately 80%[138] of the bulk of the testes),[139] whereas this is not the case for FSH, which is importantly involved.[140][141] In accordance with the fact that the testes are the source of 95% of circulating testosterone in the body, local levels of testosterone inside of the testes are extremely high, ranging from 20- to 200-fold higher than circulating concentrations.[142][54] Moreover, high levels of testosterone within the testes are required for spermatogenesis,[137] although only a small fraction (5–10%) of normal levels appears to actually be necessary for spermatogenesis.[54][143]
Unlike with antigonadotropic antiandrogens like CPA and GnRH analogues, it has been reported that bicalutamide monotherapy (at 50 mg/day) has very little or no effect on the ultrastructure of the testes and on spermatogenesis in men even after long-term therapy (>4 years).[144][145] This may be explained by the extremely high local levels of testosterone in the testes, in that it is likely that systemic bicalutamide therapy is unable to achieve concentrations of the drug within the testes that are able to considerably block androgen signaling in this part of the body.[144] This is particularly so considering that bicalutamide increases circulating testosterone levels, and by extension gonadal testosterone production, by up to two-fold in males,[79] and that only a small fraction of normal intratesticular testosterone levels, and by extension androgen action, appears to be necessary to maintain spermatogenesis.[54][143] Bicalutamide monotherapy at 50 mg/day causes no or clinically unimportant Leydig cell hyperplasia.[145][146][147]
In contrast to bicalutamide and other pure antiandrogens or NSAAs, antigonadotropic antiandrogens suppress gonadotropin secretion, which in turn diminishes testosterone production by the testes as well as the maintenance of the testes by FSH, resulting in atrophy and loss of their function.[148] As such, bicalutamide and other NSAAs may uniquely have the potential to preserve testicular function and spermatogenesis and thus male fertility relative to alternative therapies.[144][149] In accordance with this notion, a study found that prolonged, high-dose bicalutamide treatment had minimal effects on fertility in male rats.[150] However, another study found that low-dose bicalutamide administration resulted in testicular atrophy and reduced the germ cell count in the testes of male rats by almost 50%, though the rate of successful fertilization and pregnancy following mating was not assessed.[151] Additional studies found that bicalutamide decreased testes weights, altered testes histology, and decreased sperm count in male rats.[152][153][154] Yet another study found that bicalutamide has no effect on testes weights or spermatogenesis in male rats.[155]
Treatment of men with exogenous testosterone or other AAS results in suppression of gonadotropin secretion and gonadal testosterone production due to their antigonadotropic effects or activation of the AR in the pituitary gland, resulting in inhibition or abolition of spermatogenesis and fertility:[156]
Treatment of an infertile man with testosterone does [not] improve spermatogenesis, since exogenous administrated testosterone and its metabolite estrogen will suppress both GnRH production by the hypothalamus and luteinizing hormone production by the pituitary gland and subsequently suppress testicular testosterone production. Also, high levels of testosterone are needed inside the testis and this can never be accomplished by oral or parenteral administration of androgens. Suppression of testosterone production by the leydig cells will result in a deficient spermatogenesis, as can be seen in men taking anabolic–androgenic steroids.[156]
In contrast, pure AR antagonists would, in theory, result in the opposite (although reduced semen volume and sexual dysfunction may occur):[157]
It is theoretically a sound hypothesis that the spermatogenesis can be increased by indirectly stimulating FSH and LH secretions from the pituitary gland. However, for this to fructify, it requires the use of testosterone antagonist to nullify the negative feedback effect of circulating testosterone on the release of FSH and LH, thus augmenting the secretion of testosterone and spermatogenesis. Unfortunately, a testosterone antagonist will be unacceptable to males, as it may reduce secondary sexual functions including erection and ejaculation that is vital for the successful fertilization.[157]
However, while bicalutamide does not appear to adversely influence testicular spermatogenesis, and healthy sperm can be produced within the testes during bicalutamide monotherapy, AR antagonists may be able to interfere with male fertility via interference with androgen signaling beyond the testes.[158] The maturation as well as transport of sperm occurs not only in the testes but also outside of the testes in the epididymides and vas deferens, and these processes in these tissues are dependent on AR signaling similarly to testicular spermatogenesis.[158] However, whereas androgen levels are extremely high in the testes, this is not true in the epididymides and vas deferens.[158] As androgen levels are relatively low in these tissues, at least compared to the testes, bicalutamide may be able to block AR signaling in these parts of the body to an extent that is sufficient to interfere with male fertility.[158] Indeed, the AAS mesterolone has been used to improve sperm quality and fertility in men because, apparently unlike other AAS, it shows minimal antigonadotropic effects at typical clinical dosages but activates the AR and thereby supports sperm maturation in the epididymides.[159] However, this use of mesterolone is controversial and its efficacy for such purposes is not fully certain.[159]
Although bicalutamide alone would appear to have minimal detrimental effect on testicular spermatogenesis and hence on certain aspects of male fertility, other hormonal agents that bicalutamide may be combined with, including GnRH analogues and particularly estrogens (as in transgender hormone therapy), can have a considerable detrimental effect on fertility.[160][161] This is largely a consequence of their antigonadotropic activity.[161] Antigonadotropic agents like high-dose CPA,[162][163] high-dose androgens (e.g., testosterone esters), and GnRH antagonists (though notably not GnRH agonists in the case of fertility) produce hypogonadism and high rates of severe or complete infertility (e.g., severe oligospermia or complete azoospermia) in men.[161] However, these effects are fully and often rapidly reversible with their discontinuation, even after prolonged treatment.[161][163] In contrast, while estrogens at sufficiently high dosages similarly are able to produce hypogonadism and to abolish or severely impair spermatogenesis,[160] this is not necessarily reversible in the case of estrogens and can be long-lasting after prolonged exposure.[161][164] The difference is attributed to an apparently unique, direct cytotoxic and adverse effect of high concentrations of estrogens on the Leydig cells of the testes.[161][164]
Other activities
Cytochrome P450 modulation
It has been reported that bicalutamide may have the potential to inhibit the enzymes CYP3A4 and, to a lesser extent, CYP2C9, CYP2C19, and CYP2D6, based on in vitro research.[1] However, no relevant inhibition of CYP3A4 has been observed in vivo with bicalutamide at a dose of 150 mg (using midazolam as a specific marker of CYP3A4 activity).[1] In animals, bicalutamide has been found to be an inducer of certain cytochrome P450 enzymes.[1] However, dosages of 150 mg/day or less have shown no evidence of this in humans.[1]
Bicalutamide has been identified as a strong CYP27A1 (cholesterol 27-hydroxylase) inhibitor in vitro.[165] CYP27A1 converts cholesterol into 27-hydroxycholesterol, an oxysterol that has multiple biological functions including direct, tissue-specific activation of the ER (it has been characterized as a selective estrogen receptor modulator) and the liver X receptor.[165] 27-Hydroxycholesterol has been found to increase ER-positive breast cancer cell growth via its estrogenic action, and hence, it has been proposed that bicalutamide and other CYP27A1 inhibitors may be effective as adjuvant therapies to aromatase inhibitors in the treatment of ER-positive breast cancer.[165] In addition to CYP27A1, bicalutamide has been found to bind to and inhibit CYP46A1 (cholesterol 24-hydroxylase) in vitro, but this has yet to be assessed and confirmed in vivo.[166]
P-Glycoprotein inhibition
Bicalutamide, as well as enzalutamide, have been found to act as inhibitors of P-glycoprotein efflux and ATPase activity.[167][168][169] This action may reverse docetaxel resistance in prostate cancer cells by reducing transport of the drug out of these cells.[167][168][169]
GABAA receptor positive modulation
All of the NSAAs approved for the treatment of prostate cancer have been found to possess an off-target action of acting as weak non-competitive inhibitors of human GABAA receptor currents in vitro to varying extents.[170][171] The IC50 values are 44 μM for flutamide (as hydroxyflutamide), 21 μM for nilutamide, 5.2 μM for bicalutamide, and 3.6 μM for enzalutamide.[170] In addition, flutamide, nilutamide, and enzalutamide have been found to cause convulsions and/or death in mice at sufficiently high doses.[170] Bicalutamide was notably not found to do this, but this was likely simply due to the limited central nervous system penetration of bicalutamide in this species.[170] In any case, enzalutamide is the only approved NSAA that has been found to be associated with a significantly increased incidence of seizures and other associated side effects clinically, so the relevance of the aforementioned findings with regard to bicalutamide and the other NSAAs is unclear.[170]
Miscellaneous
Bicalutamide has been identified as a potent antagonist of the protease-activated receptor 2 (PAR-2) and as a ligand and inhibitor of α2-macroglobulin.[172][173]
Pharmacokinetics
| 50 mg/day | 150 mg/day | |
|---|---|---|
| Cmax | 0.77 μg/mL (1.8 μmol/L) | 1.4 μg/mL (3.3 μmol/L) |
| tmax | 31 hours | 39 hours |
| Css | 8.85 μg/mL (20.6 μmol/L) | 21.6–28.5 μg/mL (50.2–66.3 μmol/L) |
| tss | 4–12 weeks | 4–12 weeks |
| Notes: All values are for (R)-bicalutamide. Sources: [1][49] | ||
The pharmacokinetics of bicalutamide are unaffected by food, age, body weight, renal impairment, and mild-to-moderate hepatic impairment.[1][47] However, it has been observed that steady-state concentrations of bicalutamide are higher in Japanese individuals than in Caucasians, indicating that ethnicity may be associated with differences in the pharmacokinetics of bicalutamide in some instances.[1]
Absorption
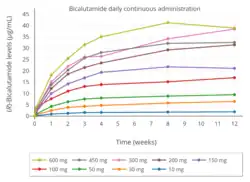
Bicalutamide is extensively and well-absorbed following oral administration,[1] and its absorption is not affected by food.[2][174] The absolute bioavailability of bicalutamide in humans is unknown due to its very low water solubility and hence lack of an assessable intravenous formulation.[1][2] However, the absolute bioavailability of bicalutamide has been found to be high in animals at low doses (109% in mice at 10 mg/kg; 72% in rats at 1 mg/kg; 100% in dogs at 0.1 mg/kg), but diminishes with increasing doses such that the bioavailability of bicalutamide is low at high doses (10% in rats at 250 mg/kg; 31% in dogs at 100 mg/kg).[1][175][176] In accordance, absorption of (R)-bicalutamide in humans is slow and extensive but saturable,[48] with steady-state levels increasing linearly at a dosage of up to 150 mg/day and non-linearly at higher dosages.[1]
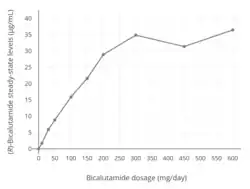
At higher dosages of 100 to 200 mg/day, absorption of bicalutamide is approximately linear, with a small but increasing departure from linearity above 150 mg/day.[177] In terms of geometric mean steady-state concentrations of (R)-bicalutamide, the departures from linearity were 4%, 13%, 17%, and 32% with dosages of 100, 150, 200, and 300 mg/day, respectively.[1] There is a plateau in steady-state levels of (R)-bicalutamide with bicalutamide dosages above 300 mg/day, and, accordingly, dosages of bicalutamide of 300 to 600 mg/day result in similar circulating concentrations of (R)-bicalutamide and similar degrees clinically of efficacy, tolerability, and toxicity.[1][178] Relative to 150 mg/day bicalutamide, levels of (R)-bicalutamide are about 15% higher at a dosage of 200 mg/day and about 50% higher at a dosage of 300 mg/day.[178] In contrast to (R)-bicalutamide, the inactive enantiomer (S)-bicalutamide is much more rapidly absorbed (as well as cleared from circulation).[1]
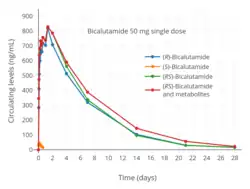
Steady-state concentrations of the drug are reached after 4 to 12 weeks of administration independently of dosage, with an approximate 10- to 20-fold progressive accumulation of circulating levels of (R)-bicalutamide.[48][179][78][47] The relatively long time to reach steady-state is a product of the long elimination half-life of bicalutamide.[47] With single 50 mg and 150 mg doses of bicalutamide, mean peak concentrations (Cmax) of (R)-bicalutamide are 0.77 μg/mL (1.8 μmol/L) (at 31 hours) and 1.4 μg/mL (3.3 μmol/L) (at 39 hours), respectively.[48][45] At steady-state, mean circulating concentrations (Css) of (R)-bicalutamide with 50 mg/day and 150 mg/day bicalutamide are 8.85 μg/mL (20.6 μmol/L) and 21.6 μg/mL (50.2 μmol/L), respectively.[48][45][46][47] In another 150 mg/day bicalutamide study, mean circulating concentrations of (R)-bicalutamide were 19.4 μg/mL (45.1 μmol/L) and 28.5 μg/mL (66.3 μmol/L) on days 28 and 84 (weeks 4 and 12) of treatment, respectively.[49]
Distribution
The apparent oral volume of distribution (VSS/F) at steady state of (R)-bicalutamide with oral administration of a single 5 to 80 mg dose of (R)-bicalutamide in a novel solid dispersion with a polymeric matrix of hydroxypropyl methylcellulose phthalate (HP55S) (also known as (R)-bicalutamide/HP55S) ranges from 22.53 ± 3.71 L to 25.38 ± 2.69 L.[180] Bicalutamide is highly protein-bound (96.1% for racemic bicalutamide, 99.6% for (R)-bicalutamide)), mainly to albumin.[3][1][181] It has negligible affinity for SHBG and no affinity for corticosteroid-binding globulin.[20]
The tissue distribution of bicalutamide is not well-characterized.[181] However, it has been reported that distribution studies with bicalutamide have shown that preferential (i.e., tissue-selective) accumulation in anabolic (e.g., muscle) tissues does not occur.[182] There are no available data on hepatic bicalutamide concentrations in humans, but a rat study found that oral bicalutamide treatment resulted in 4-fold higher concentrations of the drug in the liver relative to plasma (a common finding with orally administered drugs, due to transfer through the hepatic portal system prior to reaching circulation).[1][183] In men receiving 150 mg/day bicalutamide, concentrations of (R)-bicalutamide in semen were 4.9 μg/mL (11 μmol/L), and the amount of the drug that could potentially be delivered to a female partner during sexual intercourse is regarded as low (estimated at 0.3 μg/kg) and below the amount that is required to induce changes in the offspring of laboratory animals.[46]
Based on animal research, it was initially thought that bicalutamide was unable to cross the blood–brain barrier into the central nervous system and hence would be a peripherally-selective antiandrogen in humans.[184][26] This conclusion was drawn from the finding that bicalutamide does not increase LH or testosterone levels in multiple tested animal species (including rats and dogs).[184][26][185][186] AR antagonists like flutamide normally do this by blocking ARs in the pituitary gland and hypothalamus in the brain and thereby disinhibiting the HPG axis.[77] In humans however, bicalutamide has been found to increase LH and testosterone levels, and to a comparable extent relative to flutamide and nilutamide.[1][77][187][188] This occurs to a significant extent at even a very low dosage of 10 mg/day bicalutamide.[76] As such, it appears that there are species differences in the central penetration of bicalutamide and that the medication does indeed cross the blood–brain barrier and affect central function in humans.[1][188] This is supported by potential side effects of bicalutamide, in spite of increased testosterone levels, like hot flashes and decreased sexual interest in men.[189]
Metabolism
The metabolism of bicalutamide is hepatic and stereoselective.[3][174] The inactive (S)-enantiomer is metabolized mainly by glucuronidation and is rapidly cleared from circulation, while the active (R)-isomer is slowly hydroxylated and then glucuronidated.[174] In accordance, the active (R)-enantiomer has a far longer elimination half-life than the (S)-isomer,[22] and circulating levels of (R)-bicalutamide are 10- to 20-fold[48] and 100-fold higher than those of (S)-bicalutamide after a single dose and at steady-state, respectively.[23][24] (R)-Bicalutamide is almost exclusively metabolized via hydroxylation into (R)-hydroxybicalutamide by the cytochrome P450 enzyme CYP3A4.[1][3][23] Bicalutamide is also glucuronidated by UGT1A9, a UDP-glucuronyltransferase,[4] into bicalutamide glucuronide, and (R)-hydroxybicalutamide glucuronide is formed from the metabolism of (R)-hydroxybicalutamide by UGT1A9.[1][4][6] Similar to the inactive (S)-enantiomer of bicalutamide, (R)-hydroxybicalutamide is glucuronidated and rapidly cleared from circulation.[190] None of the metabolites of bicalutamide are known to be active.[3][5] Following administration of bicalutamide, only low concentrations of the metabolites are detectable in blood plasma, while unchanged bicalutamide predominates.[1] (R)-Bicalutamide has a long elimination half-life of 5.8 days with a single dose,[7][82] and an elimination half-life of 7 to 10 days with repeated administration, which allows for convenient once-daily dosing of bicalutamide.[8]
Elimination
Bicalutamide is eliminated in feces (43%) and urine (34%),[3][174] whereas its metabolites are eliminated in approximately equal proportions in urine and bile.[75][176] It is excreted to a substantial extent in its unmetabolized form, with both bicalutamide and its metabolites excreted mainly as glucuronide conjugates.[22]
Graphs
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Cockshott ID (2004). "Bicalutamide: clinical pharmacokinetics and metabolism". Clinical Pharmacokinetics. 43 (13): 855–878. doi:10.2165/00003088-200443130-00003. PMID 15509184.
These data indicate that direct glucuronidation is the main metabolic pathway for the rapidly cleared (S)-bicalutamide, whereas hydroxylation followed by glucuronidation is a major metabolic pathway for the slowly cleared (R)-bicalutamide.
- 1 2 3 4 Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 497, 521. ISBN 978-0-7817-2845-4. Archived from the original on 11 May 2016.
- 1 2 3 4 5 6 7 8 9 Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 121, 1288, 1290. ISBN 978-0-7817-6879-5. Archived from the original on 8 September 2017.
- 1 2 3 Grosse L, Campeau AS, Caron S, Morin FA, Meunier K, Trottier J, Caron P, Verreault M, Barbier O (August 2013). "Enantiomer selective glucuronidation of the non-steroidal pure anti-androgen bicalutamide by human liver and kidney: role of the human UDP-glucuronosyltransferase (UGT)1A9 enzyme". Basic & Clinical Pharmacology & Toxicology. 113 (2): 92–102. doi:10.1111/bcpt.12071. PMC 3815647. PMID 23527766.
- 1 2 Dole EJ, Holdsworth MT (1997). "Nilutamide: an antiandrogen for the treatment of prostate cancer". The Annals of Pharmacotherapy. 31 (1): 65–75. doi:10.1177/106002809703100112. PMID 8997470. S2CID 20347526.
page 67: Currently, information is not available regarding the activity of the major urinary metabolites of bicalutamide, bicalutamide glucuronide, and hydroxybicalutamide glucuronide.
- 1 2 Schellhammer PF (September 2002). "An evaluation of bicalutamide in the treatment of prostate cancer". Expert Opinion on Pharmacotherapy. 3 (9): 1313–28. doi:10.1517/14656566.3.9.1313. PMID 12186624. S2CID 32216411.
The clearance of bicalutamide occurs pre- dominantly by hepatic metabolism and glucuronidation, with excretion of the resulting inactive metabolites in the urine and faces.
- 1 2 Skidmore-Roth L (17 April 2013). Mosby's 2014 Nursing Drug Reference – Elsevieron VitalSource. Elsevier Health Sciences. pp. 193–194. ISBN 978-0-323-22267-9.
- 1 2 Jordan VC, Furr BJ (5 February 2010). Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. pp. 350–. ISBN 978-1-59259-152-7. Archived from the original on 29 May 2016.
- 1 2 3 4 Hamann LG, Higuchi RI, Zhi L, Edwards JP, Wang XN, Marschke KB, Kong JW, Farmer LJ, Jones TK (1998). "Synthesis and biological activity of a novel series of nonsteroidal, peripherally selective androgen receptor antagonists derived from 1,2-dihydropyridono[5,6-g]quinolines". J. Med. Chem. 41 (4): 623–39. doi:10.1021/jm970699s. PMID 9484511.
- ↑ Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T, Miyakawa M, Amano S, Sumita Y, Oguro N (November 2003). "Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis". Biol. Pharm. Bull. 26 (11): 1563–9. doi:10.1248/bpb.26.1563. PMID 14600402.
- 1 2 Nagata N, Miyakawa M, Amano S, Furuya K, Yamamoto N, Nejishima H, Inoguchi K (2011). "Tetrahydroquinolines as a novel series of nonsteroidal selective androgen receptor modulators: structural requirements for better physicochemical and biological properties". Bioorg. Med. Chem. Lett. 21 (21): 6310–3. doi:10.1016/j.bmcl.2011.08.118. PMID 21944856.
- 1 2 Nagata N, Miyakawa M, Amano S, Furuya K, Yamamoto N, Inoguchi K (2011). "Design and synthesis of tricyclic tetrahydroquinolines as a new series of nonsteroidal selective androgen receptor modulators (SARMs)". Bioorg. Med. Chem. Lett. 21 (6): 1744–7. doi:10.1016/j.bmcl.2011.01.073. PMID 21349712.
- 1 2 Kinoyama I, Taniguchi N, Toyoshima A, Nozawa E, Kamikubo T, Imamura M, Matsuhisa A, Samizu K, Kawanimani E, Niimi T, Hamada N, Koutoku H, Furutani T, Kudoh M, Okada M, Ohta M, Tsukamoto S (2006). "(+)-(2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-dimethyl-N-[6-(trifluoromethyl)pyridin-3- yl]piperazine-1-carboxamide (YM580) as an orally potent and peripherally selective nonsteroidal androgen receptor antagonist". J. Med. Chem. 49 (2): 716–26. doi:10.1021/jm050293c. PMID 16420057.
- ↑ Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT (April 2005). "Structural basis for antagonism and resistance of bicalutamide in prostate cancer". Proceedings of the National Academy of Sciences of the United States of America. 102 (17): 6201–6. Bibcode:2005PNAS..102.6201B. doi:10.1073/pnas.0500381102. PMC 1087923. PMID 15833816.
- 1 2 Ayub M, Levell MJ (August 1989). "The effect of ketoconazole related imidazole drugs and antiandrogens on [3H] R 1881 binding to the prostatic androgen receptor and [3H]5 alpha-dihydrotestosterone and [3H]cortisol binding to plasma proteins". J. Steroid Biochem. 33 (2): 251–5. doi:10.1016/0022-4731(89)90301-4. PMID 2788775.
- 1 2 Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- ↑ Balaj K (25 April 2016). Managing Metastatic Prostate Cancer In Your Urological Oncology Practice. Springer. pp. 24–25. ISBN 978-3-319-31341-2. Archived from the original on 8 September 2017.
- ↑ Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP (July 2002). "Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor". The Journal of Biological Chemistry. 277 (29): 26321–6. doi:10.1074/jbc.M203310200. PMID 12015321.
- 1 2 3 4 Denis L (6 December 2012). Antiandrogens in Prostate Cancer: A Key to Tailored Endocrine Treatment. Springer Science & Business Media. pp. 128, 158, 203, 231–232. ISBN 978-3-642-45745-6.
At daily doses of 10, 30 and 50 mg decreases of this range were noted in 33%, 53% and 83% of treated patients. [% PSA decline: 10 mg: 57%; 30 mg: 73%; 50 mg: 90%; 100 mg: 97%; 150 mg: 97%; CAS: 97%.]
- 1 2 3 4 Furr BJ (June 1995). "Casodex: preclinical studies and controversies". Annals of the New York Academy of Sciences. 761 (1): 79–96. Bibcode:1995NYASA.761...79F. doi:10.1111/j.1749-6632.1995.tb31371.x. PMID 7625752. S2CID 37242269.
It is suggested that the resulting increase in output of androgen by the testis requires an increased dose of antiandrogen to neutralize any stimulatory effect on the prostate gland. This is a controversial topic [...], but when given in combination with medical or surgical castration, any rise in serum LH becomes irrelevant to clinical outcome.
- ↑ Waller AS, Sharrard RM, Berthon P, Maitland NJ (June 2000). "Androgen receptor localisation and turnover in human prostate epithelium treated with the antiandrogen, casodex". Journal of Molecular Endocrinology. 24 (3): 339–51. CiteSeerX 10.1.1.499.7722. doi:10.1677/jme.0.0240339. PMID 10828827.
- 1 2 3 4 5 Schellens JH, McLeod HL, Newell DR (5 May 2005). Cancer Clinical Pharmacology. OUP Oxford. pp. 229–230. ISBN 978-0-19-262966-1. Archived from the original on 10 June 2016.
- 1 2 3 Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1372–1373. ISBN 978-1-60913-345-0. Archived from the original on 3 May 2016.
- 1 2 Butler SK, Govindan R (25 October 2010). Essential Cancer Pharmacology: The Prescriber's Guide. Lippincott Williams & Wilkins. pp. 49–. ISBN 978-1-60913-704-5.
- 1 2 3 4 Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1119, 1196, 1208. ISBN 978-0-7817-1750-2. Archived from the original on 8 September 2017.
- 1 2 3 4 Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- ↑ Bagatelle C, Bremner WJ (27 May 2003). Androgens in Health and Disease. Springer Science & Business Media. pp. 25–. ISBN 978-1-59259-388-0.
- 1 2 3 4 5 Ito Y, Sadar MD (2018). "Enzalutamide and blocking androgen receptor in advanced prostate cancer: lessons learnt from the history of drug development of antiandrogens". Res Rep Urol. 10: 23–32. doi:10.2147/RRU.S157116. PMC 5818862. PMID 29497605.
- 1 2 Guise TA, Oefelein MG, Eastham JA, Cookson MS, Higano CS, Smith MR (2007). "Estrogenic side effects of androgen deprivation therapy". Reviews in Urology. 9 (4): 163–80. PMC 2213888. PMID 18231613.
The incidence of gynecomastia varies with the type and duration of ADT.65,83 For example, it is reported in 40% to 80% of men on estrogen therapy (eg, DES), 40% to 70% of men on antiandrogens (bicalutamide, flutamide, or nilutamide, including > 50% with high-dose bicalutamide [150 mg]), 25% of men on combined androgen blockade (LHRH with an antiandrogen), and 10% to 15% of men on LHRH alone or after orchiectomy.66
- ↑ Bulldan A, Malviya VN, Upmanyu N, Konrad L, Scheiner-Bobis G (2017). "Testosterone/bicalutamide antagonism at the predicted extracellular androgen binding site of ZIP9". Biochim. Biophys. Acta. 1864 (12): 2402–2414. doi:10.1016/j.bbamcr.2017.09.012. PMID 28943399.
- ↑ Thomas P, Converse A, Berg HA (2017). "ZIP9, a novel membrane androgen receptor and zinc transporter protein". Gen. Comp. Endocrinol. 257: 130–136. doi:10.1016/j.ygcen.2017.04.016. PMID 28479083.
- ↑ Pi M, Parrill AL, Quarles LD (2010). "GPRC6A mediates the non-genomic effects of steroids". J. Biol. Chem. 285 (51): 39953–64. doi:10.1074/jbc.M110.158063. PMC 3000977. PMID 20947496.
- ↑ Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H (June 1987). "ICI 176,334: a novel non-steroidal, peripherally selective antiandrogen". J. Endocrinol. 113 (3): R7–9. doi:10.1677/joe.0.113R007. PMID 3625091.
- ↑ Teutsch G, Goubet F, Battmann T, Bonfils A, Bouchoux F, Cerede E, Gofflo D, Gaillard-Kelly M, Philibert D (January 1994). "Non-steroidal antiandrogens: synthesis and biological profile of high-affinity ligands for the androgen receptor". J. Steroid Biochem. Mol. Biol. 48 (1): 111–9. doi:10.1016/0960-0760(94)90257-7. PMID 8136296.
- ↑ Winneker RC, Wagner MM, Batzold FH (December 1989). "Studies on the mechanism of action of Win 49596: a steroidal androgen receptor antagonist". J. Steroid Biochem. 33 (6): 1133–8. doi:10.1016/0022-4731(89)90420-2. PMID 2615358.
- 1 2 Luo, S; Martel, C; LeBlanc, G; Candas, B; Singh, S M; Labrie, C; Simard, J; Belanger, A; Labrie, F (1996). "Relative potencies of Flutamide and Casodex: preclinical studies". Endocrine Related Cancer. 3 (3): 229–241. doi:10.1677/erc.0.0030229. ISSN 1351-0088.
- ↑ Kemppainen JA, Wilson EM (July 1996). "Agonist and antagonist activities of hydroxyflutamide and Casodex relate to androgen receptor stabilization". Urology. 48 (1): 157–63. doi:10.1016/S0090-4295(96)00117-3. PMID 8693644.
- ↑ Furr BJ (2009). "Research on reproductive medicine in the pharmaceutical industry". Human Fertility. 1 (1): 56–63. doi:10.1080/1464727982000198131. PMID 11844311.
- ↑ Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL (2009). "Development of a second-generation antiandrogen for treatment of advanced prostate cancer". Science. 324 (5928): 787–90. Bibcode:2009Sci...324..787T. doi:10.1126/science.1168175. PMC 2981508. PMID 19359544.
[...] bicalutamide has relatively low affinity for AR (at least 30-fold reduced relative to the natural ligand dihydrotestosterone (DHT)) (7), [...]
- ↑ Furr, B J A (1997). "Relative potencies of flutamide and 'Casodex'". Endocrine-Related Cancer. 4 (2): 197–202. doi:10.1677/erc.0.0040197. ISSN 1351-0088.
'Casodex' has been used in both models; in the Shionogi mammary tumour, an independent study by Darbre & King (1990) shows that a 1000-fold excess of 'Casodex' completely inhibits the response to 5α-DHT. Inspection of the data shown by Luo et al. suggests that at least a 10 000-fold excess of 'Casodex' and around a 3500-fold excess of hydroxyflutamide is required to achieve complete inhibition of 5α-DHT-stimulated growth.
- ↑ Furr BJ (1996). "The development of Casodex (bicalutamide): preclinical studies". European Urology. 29 Suppl 2 (2): 83–95. doi:10.1159/000473846. PMID 8717469.
- 1 2 Figg W, Chau CH, Small EJ (14 September 2010). Drug Management of Prostate Cancer. Springer Science & Business Media. pp. 56, 71–72, 75, 93. ISBN 978-1-60327-829-4.
- ↑ Chapple CR, Steers WD (10 May 2011). Practical Urology: Essential Principles and Practice: Essential Principles and Practice. Springer Science & Business Media. pp. 225–. ISBN 978-1-84882-034-0.
Normal reference ranges for serum total testosterone in adult men is generally considered to be 300–1,000 ng/dL (10–35 nmol/L).
- ↑ Gentile V, Panebianco V, Sciarra A (11 April 2014). Multidisciplinary Management of Prostate Cancer: The Role of the Prostate Cancer Unit. Springer Science & Business Media. pp. 106–. ISBN 978-3-319-04385-2.
The standard castrate level is <50 ng/dl. It was defined more than 40 years ago, when testosterone level testing was limited. However, current testing methods using chemiluminescence have found that the mean value of testosterone after surgical castration is 15 ng/dL.
- 1 2 3 "Casodex® (bicalutamide) Tablets" (PDF). FDA. Archived (PDF) from the original on 27 February 2017.
- 1 2 3 "COSUDEX® (bicalutamide) 150 mg tablets". TGA. Archived from the original on 14 September 2016.
- 1 2 3 4 5 Denis L, Mahler C (January 1996). "Pharmacodynamics and pharmacokinetics of bicalutamide: defining an active dosing regimen". Urology. 47 (1A Suppl): 26–8, discussion 29–32. doi:10.1016/S0090-4295(96)80004-5. PMID 8560674.
- 1 2 3 4 5 6 Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer" (PDF). Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. S2CID 46966712. Archived from the original (PDF) on 28 August 2016. Retrieved 23 November 2017.
- 1 2 3 Boccardo F, Rubagotti A, Conti G, Potenzoni D, Manganelli A, Del Monaco D (2005). "Exploratory study of drug plasma levels during bicalutamide 150 mg therapy co-administered with tamoxifen or anastrozole for prophylaxis of gynecomastia and breast pain in men with prostate cancer". Cancer Chemotherapy and Pharmacology. 56 (4): 415–20. doi:10.1007/s00280-005-1016-1. PMID 15838655. S2CID 23014567.
- 1 2 3 Kolvenbag GJ, Nash A (April 1999). "Bicalutamide dosages used in the treatment of prostate cancer". Prostate. 39 (1): 47–53. doi:10.1002/(SICI)1097-0045(19990401)39:1<47::AID-PROS8>3.0.CO;2-X. PMID 10221266.
- 1 2 Chung LW, Isaacs WB, Simons JW (10 November 2007). Prostate Cancer: Biology, Genetics, and the New Therapeutics. Springer Science & Business Media. pp. 365–. ISBN 978-1-59745-224-3. Archived from the original on 20 May 2016.
- 1 2 3 Melmed S, Polonsky KS, Reed Larsen P, Kronenberg HM (30 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 704–708, 711, 1104. ISBN 978-0-323-29738-7.
- ↑ Bruskewitz R (6 December 2012). Atlas of the Prostate. Springer Science & Business Media. pp. 5, 190. ISBN 978-1-4615-6505-5.
- 1 2 3 4 Nieschlag E, Behre HM (6 December 2012). Testosterone: Action – Deficiency – Substitution. Springer Science & Business Media. pp. 130, 276. ISBN 978-3-642-72185-4.
- ↑ Mozayani A, Raymon L (18 September 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
- 1 2 Chabner BA, Longo DL (8 November 2010). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. pp. 679–680. ISBN 978-1-60547-431-1.
From a structural standpoint, antiandrogens are classified as steroidal, including cyproterone [acetate] (Androcur) and megestrol [acetate], or nonsteroidal, including flutamide (Eulexin, others), bicalutamide (Casodex), and nilutamide (Nilandron). The steroidal antiandrogens are rarely used.
- ↑ Mydlo JH, Godec CJ (29 September 2015). Prostate Cancer: Science and Clinical Practice. Elsevier Science. pp. 516–521, 534–540. ISBN 978-0-12-800592-7. Archived from the original on 8 September 2017.
- 1 2 3 Strauss III JF, Barbieri RL (28 August 2013). Yen & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 688–. ISBN 978-1-4557-5972-9.
Bone density improves in men receiving bicalutamide, most likely secondary to the 146% increase in estradiol and the fact that estradiol is the major mediator of bone density in men.
- ↑ Luo S, Martel C, Chen C, Labrie C, Candas B, Singh SM, Labrie F (December 1997). "Daily dosing with flutamide or Casodex exerts maximal antiandrogenic activity". Urology. 50 (6): 913–9. doi:10.1016/S0090-4295(97)00393-2. PMID 9426723.
- ↑ Wirth MP, Hakenberg OW, Froehner M (February 2007). "Antiandrogens in the treatment of prostate cancer". European Urology. 51 (2): 306–13, discussion 314. doi:10.1016/j.eururo.2006.08.043. PMID 17007995.
- ↑ Labrie F (January 2015). "Combined blockade of testicular and locally made androgens in prostate cancer: a highly significant medical progress based upon intracrinology". J. Steroid Biochem. Mol. Biol. 145: 144–56. doi:10.1016/j.jsbmb.2014.05.012. PMID 24925260. S2CID 23102323.
- 1 2 Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, Denis L, Iversen P, Eisenberger MA, Labrie F (May 2018). "Androgen Receptor-Targeted Treatments for Prostate Cancer: 35 Years' Progress with Antiandrogens". J. Urol. 200 (5): 956–966. doi:10.1016/j.juro.2018.04.083. PMID 29730201. S2CID 19162538.
- ↑ Student, Sebastian; Hejmo, Tomasz; Poterała-Hejmo, Aleksandra; Leśniak, Aleksandra; Bułdak, Rafał (2019). "Anti-androgen hormonal therapy for cancer and other diseases". European Journal of Pharmacology. 866: 172783. doi:10.1016/j.ejphar.2019.172783. ISSN 0014-2999. PMID 31712062.
- ↑ Nadal R, Bellmunt J (March 2016). "The evolving role of enzalutamide on the treatment of prostate cancer". Future Oncol. 12 (5): 607–16. doi:10.2217/fon.15.351. PMC 5551941. PMID 26839021.
- 1 2 Stein MN, Jang TL (June 2016). "Striving Toward a Cure for Prostate Cancer". J. Clin. Oncol. 34 (18): 2075–8. doi:10.1200/JCO.2015.66.3146. PMID 27022121.
- ↑ Styne, D. M. (6 December 2019). "Physiology and Disorders of Puberty". In Melmed, S.; Koenig, R. J.; Rosen, C. J.; Auchus, R. J.; Goldfine, A. B.; Williams, R. H. (eds.). Williams Textbook of Endocrinology (14 ed.). Philadelphia, PA: Elsevier. pp. 1023–1164. ISBN 9780323555968.
- 1 2 3 Marcus R, Feldman D, Nelson D, Rosen CJ (8 November 2007). Osteoporosis. Academic Press. pp. 1354–. ISBN 978-0-08-055347-4. Archived from the original on 11 June 2016.
- 1 2 Diamanti-Kandarakis E, Nestler JE, Pandas D, Pasquale R (21 December 2009). Insulin Resistance and Polycystic Ovarian Syndrome: Pathogenesis, Evaluation, and Treatment. Springer Science & Business Media. pp. 75–. ISBN 978-1-59745-310-3. Archived from the original on 19 May 2016.
- 1 2 Carrell DT, Peterson CM (23 March 2010). Reproductive Endocrinology and Infertility: Integrating Modern Clinical and Laboratory Practice. Springer Science & Business Media. pp. 163–. ISBN 978-1-4419-1436-1. Archived from the original on 4 July 2014.
- 1 2 3 Bouchard P, Caraty A (15 November 1993). GnRH, GnRH Analogs, Gonadotropins and Gonadal Peptides. CRC Press. pp. 455–456. ISBN 978-0-203-09205-7.
[...] when male levels of androgens are achieved in plasma, their effects on gonadotropin secretion are similar in women and men. [...] administration of flutamide in a group of normally-cycling women produced a clinical improvement of acne and hirsutism without any significant hormonal change. [...] All these data emphasize that physiological levels of androgens have no action on the regulation of gonadotropins in normal women. [...] Androgens do not directly play a role in gonadotropin regulation [in women].
- ↑ Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B (22 January 2009). Evidence-Based Dermatology. John Wiley & Sons. pp. 529–. ISBN 978-1-4443-0017-8. Archived from the original on 2 May 2016.
- ↑ Erem C (2013). "Update on idiopathic hirsutism: diagnosis and treatment". Acta Clinica Belgica. 68 (4): 268–74. doi:10.2143/ACB.3267. PMID 24455796. S2CID 39120534.
- ↑ Costanzo Giulio Moretti, Laura Guccione, Paola Di Giacinto, Amalia Cannuccia, Chiara Meleca, Giulia Lanzolla, Aikaterini Andreadi, Davide Lauro (2016), Efficacy and Safety of Myo-Inositol Supplementation in the Treatment of Obese Hirsute PCOS Women: Comparative Evaluation with OCP+Bicalutamide Therapy, doi:10.1210/endo-meetings.2016.RE.5.SUN-153 (inactive 31 October 2021)
{{citation}}: CS1 maint: DOI inactive as of October 2021 (link) CS1 maint: uses authors parameter (link) - 1 2 3 Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clinical Pharmacokinetics. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622. S2CID 25200595.
- 1 2 Fradet Y (February 2004). "Bicalutamide (Casodex) in the treatment of prostate cancer". Expert Review of Anticancer Therapy. 4 (1): 37–48. doi:10.1586/14737140.4.1.37. PMID 14748655. S2CID 34153031.
In contrast, the incidence of diarrhea was comparable between the bicalutamide and placebo groups (6.3 vs. 6.4%, respectively) in the EPC program [71].
- 1 2 3 4 5 6 7 8 Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID (1998). "Casodex 10-200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. An overview of the efficacy, tolerability and pharmacokinetics from three phase II dose-ranging studies. Casodex Study Group". Eur. Urol. 33 (1): 39–53. doi:10.1159/000019526. PMID 9471040. S2CID 71758492.
- 1 2 3 4 Iversen P, Melezinek I, Schmidt A (January 2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992. S2CID 28215804.
- 1 2 DeVita Jr VT, Lawrence TS, Rosenberg SA (7 January 2015). DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. Wolters Kluwer Health. pp. 1142–. ISBN 978-1-4698-9455-3.
- 1 2 Eri LM, Haug E, Tveter KJ (March 1995). "Effects on the endocrine system of long-term treatment with the non-steroidal anti-androgen Casodex in patients with benign prostatic hyperplasia". British Journal of Urology. 75 (3): 335–40. doi:10.1111/j.1464-410X.1995.tb07345.x. PMID 7537602.
- ↑ Verhelst J, Denis L, Van Vliet P, Van Poppel H, Braeckman J, Van Cangh P, Mattelaer J, D'Hulster D, Mahler C (October 1994). "Endocrine profiles during administration of the new non-steroidal anti-androgen Casodex in prostate cancer". Clin. Endocrinol. (Oxf). 41 (4): 525–30. doi:10.1111/j.1365-2265.1994.tb02585.x. PMID 7525125. S2CID 7880831.
- 1 2 Wadhwa VK, Weston R, Parr NJ (June 2011). "Bicalutamide monotherapy preserves bone mineral density, muscle strength and has significant health-related quality of life benefits for osteoporotic men with prostate cancer". BJU Int. 107 (12): 1923–9. doi:10.1111/j.1464-410X.2010.09726.x. PMID 20950306. S2CID 205543615.
- 1 2 Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–2939, 2946. ISBN 978-1-4160-6911-9. Archived from the original on 5 May 2016.
- ↑ Lunglmayr G (1989). "Casodex (ICI 176,334), a new, non-steroidal anti-androgen. Early clinical results". Hormone Research. 32 Suppl 1: 77–81. doi:10.1159/000181316. PMID 2515147.
- ↑ E.E. Müller (6 December 2012). Peptides and Non Peptides of Oncologic and Neuroendocrine Relevance: From Basic to Clinical Research. Springer Science & Business Media. pp. 231–. ISBN 978-88-470-2085-6.
Pure anti-androgens can be given as monotherapy in the attempt to avoid the side effects caused by androgen-suppressive therapies (loss of libido, impotency, osteoporosis, pathological fractures, decrease of muscle mass and tone, progressive anaemia, asthenia, and depression) (Tyrrell, 1992). The use of these compounds in patients with intact gonads induces a condition of hypergonadotrophic hypergonadism, which allows high circulating levels of testosterone to be maintained.
- ↑ Knuth UA, Hano R, Nieschlag E (November 1984). "Effect of flutamide or cyproterone acetate on pituitary and testicular hormones in normal men". J. Clin. Endocrinol. Metab. 59 (5): 963–9. doi:10.1210/jcem-59-5-963. PMID 6237116.
Since FLU is devoid of intrinsic hormonal activity, its antiandrogenic property leads to increased serum testosterone (T) levels and elevated gonadotropin values. The effect of this unique endocrine situation, which may be described as "hypergonadotropic hypergonadism."
- ↑ Müderris II, Bayram F, Ozçelik B, Güven M (February 2002). "New alternative treatment in hirsutism: bicalutamide 25 mg/day". Gynecol. Endocrinol. 16 (1): 63–6. doi:10.1080/gye.16.1.63.66. PMID 11915584. S2CID 6942048.
- ↑ Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A (March 2018). "Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial". J. Clin. Endocrinol. Metab. 103 (3): 824–838. doi:10.1210/jc.2017-01186. PMID 29211888.
- ↑ Bahceci M, Tuzcu A, Canoruc N, Tuzun Y, Kidir V, Aslan C (2004). "Serum C-reactive protein (CRP) levels and insulin resistance in non-obese women with polycystic ovarian syndrome, and effect of bicalutamide on hirsutism, CRP levels and insulin resistance". Horm. Res. 62 (6): 283–7. doi:10.1159/000081973. PMID 15542929. S2CID 46261843.
- ↑ Shlomo Melmed (1 January 2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 752–. ISBN 978-0-323-29738-7.
GnRH analogues, both agonists and antagonists, severely suppress endogenous gonadotropin and testosterone production [...] Administration of GnRH agonists (e.g., leuprolide, goserelin) produces an initial stimulation of gonadotropin and testosterone secretion (known as a "flare"), which is followed in 1 to 2 weeks by GnRH receptor downregulation and marked suppression of gonadotropins and testosterone to castration levels. [...] To prevent the potential complications associated with the testosterone flare, AR antagonists (e.g., bicalutamide) are usually coadministered with a GnRH agonist for men with metastatic prostate cancer.399
- ↑ Asscheman H, Gooren LJ, Peereboom-Wynia JD (1989). "Reduction in undesired sexual hair growth with anandron in male-to-female transsexuals—experiences with a novel androgen receptor blocker". Clinical and Experimental Dermatology. 14 (5): 361–3. doi:10.1111/j.1365-2230.1989.tb02585.x. PMID 2612040. S2CID 45303518.
- ↑ Rao BR, de Voogt HJ, Geldof AA, Gooren LJ, Bouman FG (1988). "Merits and considerations in the use of anti-androgen". Journal of Steroid Biochemistry. 31 (4B): 731–7. doi:10.1016/0022-4731(88)90024-6. PMID 3143862.
- 1 2 Jameson JL, de Kretser DM, Marshall JC, De Groot LJ (7 May 2013). Endocrinology Adult and Pediatric: Reproductive Endocrinology. Elsevier Health Sciences. ISBN 978-0-323-22152-8. Archived from the original on 25 July 2014.
Nonsteroidal antiandrogens (e.g., flutamide and nilutamide) are also used, but they increase gonadotropin secretion, causing increased secretion of testosterone and estradiol.119 The latter is desirable in this context, as it has feminizing effects.
- ↑ Bach PV, Najari BB, Kashanian JA (2016). "Adjunct Management of Male Hypogonadism". Current Sexual Health Reports. 8 (4): 231–239. doi:10.1007/s11930-016-0089-7. ISSN 1548-3584. S2CID 79220716.
- ↑ Santen RJ, Leonard JM, Sherins RJ, Gandy HM, Paulsen CA (1971). "Short- and long-term effects of clomiphene citrate on the pituitary-testicular axis". J. Clin. Endocrinol. Metab. 33 (6): 970–9. doi:10.1210/jcem-33-6-970. PMID 5135636.
Increase in serum LH levels ranged from 200–700% during the initial 21 days of clomiphene administration but then plateaued. Serum FSH levels exhibited a similar plateau after 35 days, with maximum titers 70–360% over control. The range in serum testosterone increments after 7 and 51 days of clomiphene administration was similar to that observed in serum gonadotrophin levels.
- ↑ Martini L (2 December 2012). Clinical Neuroendocrinology. Elsevier. p. 239. ISBN 978-0-323-14429-2.
From the studies of Santen et al. (1971), it seems that a longer period of administration (51 days in their study) would cause an even greater rise in FSH and LH (70–360% and 200–700%, respectively).
- ↑ Sieber PR (December 2007). "Treatment of bicalutamide-induced breast events". Expert Review of Anticancer Therapy. 7 (12): 1773–9. doi:10.1586/14737140.7.12.1773. PMID 18062751. S2CID 40410461.
- 1 2 3 Kennealey GT, Furr BJ (February 1991). "Use of the nonsteroidal anti-androgen Casodex in advanced prostatic carcinoma". Urol. Clin. North Am. 18 (1): 99–110. doi:10.1016/S0094-0143(21)01397-5. PMID 1992575.
- 1 2 3 Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". The Journal of Urology. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- 1 2 Motofei IG, Rowland DL, Popa F, Kreienkamp D, Paunica S (July 2011). "Preliminary study with bicalutamide in heterosexual and homosexual patients with prostate cancer: a possible implication of androgens in male homosexual arousal". BJU International. 108 (1): 110–5. doi:10.1111/j.1464-410X.2010.09764.x. PMID 20955264. S2CID 45482984.
- 1 2 Wibowo E, Wassersug RJ (September 2013). "The effect of estrogen on the sexual interest of castrated males: Implications to prostate cancer patients on androgen-deprivation therapy". Critical Reviews in Oncology/Hematology. 87 (3): 224–38. doi:10.1016/j.critrevonc.2013.01.006. PMID 23484454.
- ↑ Simpson ER, Jones ME (2007). "Of mice and men: the many guises of estrogens". Tissue-Specific Estrogen Action. Ernst Schering Foundation Symposium Proceedings. Vol. 2006/1. pp. 45–67. doi:10.1007/2789_2006_016. ISBN 978-3-540-49547-5. PMID 17824171.
- 1 2 King SR (2008). "Emerging roles for neurosteroids in sexual behavior and function". Journal of Andrology. 29 (5): 524–33. doi:10.2164/jandrol.108.005660. PMID 18567641.
- 1 2 Morali G, Oropeza MV, Lemus AE, Perez-Palacios G (September 1994). "Mechanisms regulating male sexual behavior in the rat: role of 3 alpha- and 3 beta-androstanediols". Biology of Reproduction. 51 (3): 562–71. doi:10.1095/biolreprod51.3.562. PMID 7803627.
- ↑ Sánchez Montoya EL, Hernández L, Barreto-Estrada JL, Ortiz JG, Jorge JC (November 2010). "The testosterone metabolite 3α-diol enhances female rat sexual motivation when infused in the nucleus accumbens shell". The Journal of Sexual Medicine. 7 (11): 3598–609. doi:10.1111/j.1743-6109.2010.01937.x. PMC 4360968. PMID 20646182.
- 1 2 Chedrese PJ (13 June 2009). Reproductive Endocrinology: A Molecular Approach. Springer Science & Business Media. pp. 233–. ISBN 978-0-387-88186-7. Archived from the original on 5 September 2017.
- ↑ Frye CA, Edinger KL, Lephart ED, Walf AA (2010). "3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats". Frontiers in Aging Neuroscience. 2: 15. doi:10.3389/fnagi.2010.00015. PMC 2874398. PMID 20552051.
- ↑ Huang Q, Zhu H, Fischer DF, Zhou JN (June 2008). "An estrogenic effect of 5alpha-androstane-3beta, 17beta-diol on the behavioral response to stress and on CRH regulation". Neuropharmacology. 54 (8): 1233–8. doi:10.1016/j.neuropharm.2008.03.016. PMID 18457850. S2CID 9052079.
- ↑ Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA (November 2008). "Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice". Hormones and Behavior. 54 (5): 726–34. doi:10.1016/j.yhbeh.2008.07.013. PMC 3623974. PMID 18775724.
- ↑ Gaudet M, Vigneault É, Foster W, Meyer F, Martin AG (January 2016). "Randomized non-inferiority trial of Bicalutamide and Dutasteride versus LHRH agonists for prostate volume reduction prior to I-125 permanent implant brachytherapy for prostate cancer". Radiother Oncol. 118 (1): 141–7. doi:10.1016/j.radonc.2015.11.022. PMID 26702991.
Dutasteride and Bicalutamide is a regimen of non-inferior efficacy to LHRH agonist based regimens for prostate volume reduction prior to permanent implant prostate brachytherapy. D + B has less sexual toxicity compared to LHRH agonists prior to implant and for the first 6 months after implant. D + B is therefore an option to be considered for prostate volume reduction prior to PIPB.
- 1 2 Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- ↑ Kolvenbag, G. J. C. M. (2001). "The Prostate Cancer Continuum: The Role of Bicalutamide (CasodexR)". The Prostate Journal. 3 (1): 2–13. doi:10.1046/j.1525-1411.2001.003001002.x. ISSN 1095-5100.
- ↑ Zhang X, Li X, Allan GF, Sbriscia T, Linton O, Lundeen SG, Sui Z (August 2007). "Design, synthesis, and in vivo SAR of a novel series of pyrazolines as potent selective androgen receptor modulators". J. Med. Chem. 50 (16): 3857–69. doi:10.1021/jm0613976. PMID 17636947.
For comparison, the androgen antagonists flutamide and bicalutamide reduced both prostate and levator ani weights by 51 to 67% at 30 mg/kg.
- ↑ Ikeda, Kazuhiro; Horie-Inoue, Kuniko; Inoue, Satoshi (2019). "Functions of estrogen and estrogen receptor signaling on skeletal muscle". The Journal of Steroid Biochemistry and Molecular Biology. 191: 105375. doi:10.1016/j.jsbmb.2019.105375. ISSN 0960-0760. PMID 31067490. S2CID 145024087.
- 1 2 3 Bambury RM, Scher HI (June 2015). "Enzalutamide: Development from bench to bedside". Urologic Oncology. 33 (6): 280–8. doi:10.1016/j.urolonc.2014.12.017. PMID 25797385.
- ↑ Bambury RM, Rathkopf DE (August 2016). "Novel and next-generation androgen receptor-directed therapies for prostate cancer: Beyond abiraterone and enzalutamide". Urologic Oncology. 34 (8): 348–55. doi:10.1016/j.urolonc.2015.05.025. PMID 26162486.
- 1 2 Pinto Á (February 2014). "Beyond abiraterone: new hormonal therapies for metastatic castration-resistant prostate cancer". Cancer Biology & Therapy. 15 (2): 149–55. doi:10.4161/cbt.26724. PMC 3928129. PMID 24100689.
- ↑ Zanardi S, Puntoni M, Maffezzini M, Bandelloni R, Branchi D, Argusti A, Campodonico F, Turbino L, Mori M, Decensi A (1 December 2006). "A biomarker trial of intermittent, low-dose bicalutamide in subjects at high risk for prostate cancer: Updated results". Cancer Epidemiol Biomarkers Prev. 15 (12 Supplement): A145.
Testosterone (T), LH, E2 and SHBG levels increased on Bic, although only T changes on both doses and LH changes on Bic 100 mg were significantly different to controls (p<0.001). Changes in circulating hormones and biomarkers were evident after 3 months, and persisted through the 6th month of treatment. No change in circulating hormones or biomarkers was observed in the control group. [...] Treatment was well tolerated, although breast pain was recorded in 0/19 (0%), 8/25 (32%) and 14/22 (64%), and gynecomastia in 0/19 (0%), 11/25 (44%) and 11/22 (50%) of subjects on no treatment, Bic 50 or 100 mg, respectively.
- ↑ Zanardi, S.; Puntoni, M.; Maffezzini, M.; Bandelloni, R.; Mori, M.; Argusti, A.; Campodonico, F.; Turbino, L.; Branchi, D.; Montironi, R.; Decensi, A. (2009). "Phase I-II Trial of Weekly Bicalutamide in Men with Elevated Prostate-Specific Antigen and Negative Prostate Biopsies". Cancer Prevention Research. 2 (4): 377–384. doi:10.1158/1940-6207.CAPR-08-0205. ISSN 1940-6207. PMID 19336728.
- ↑ Decensi A, Zanardi S, Puntoni M, Bandelloni R, Branchi D, Argusti A, Campodonico F, Turbino L, Mori M, Maffezzini M (20 June 2007). "Phase I-II trial of weekly bicalutamide in men with high PSA and negative biopsy". Journal of Clinical Oncology. 25 (18_suppl): 1500. doi:10.1200/jco.2007.25.18_suppl.1500.
T, LH, estradiol and SHBG increased on Bic by 50–60%. [...] Treatment was well tolerated, mild (G1) breast pain and gynecomastia being recorded in 40% of treated subjects.
- 1 2 Wierckx K, Gooren L, T'Sjoen G (May 2014). "Clinical review: Breast development in trans women receiving cross-sex hormones". The Journal of Sexual Medicine. 11 (5): 1240–7. doi:10.1111/jsm.12487. PMID 24618412.
Other agents with anti-androgenic properties used [in the treatment of transgender women] are nonsteroidal androgen receptor blockers, such as flutamide and bicalutamide [...]
- ↑ Orentreich N, Durr NP (1974). "Mammogenesis in Transsexuals". Journal of Investigative Dermatology. 63 (1): 142–6. doi:10.1111/1523-1747.ep12678272. PMID 4365991.
- ↑ Anderson J (2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU Int. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397. S2CID 8639102.
Gynaecomastia is an expected pharmacological effect of nonsteroidal antiandrogen monotherapy and is caused by an imbalance in the normal ratio of androgenic and oestrogenic activity [32]. This is a result of an increase in oestrogen levels (after peripheral aromatization of elevated serum testosterone levels) and direct antagonism of androgen action in breast tissue.
- 1 2 Jameson JL, De Groot LJ (25 February 2015). Edndocrinology: Adult and Pediatric. Elsevier Health Sciences. pp. 2425–2426, 2139. ISBN 978-0-323-32195-2.
- 1 2 Strauss III JF, Barbieri RL (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 236–237. ISBN 978-1-4557-2758-2. Archived from the original on 14 January 2017.
- ↑ Wilson CB, Nizet V, Maldonado Y, Klein JO, Remington JS (2015). Remington and Klein's Infectious Diseases of the Fetus and Newborn Infant. Elsevier Health Sciences. pp. 190–. ISBN 978-0-323-24147-2. Archived from the original on 14 January 2017.
- ↑ Yen SS, Jaffe RB, Barbieri RL (2001). Endocrinología de la reproducción: fisiología, fisiopatología y manejo clínico. Ed. Medic Panamericana. pp. 303–. ISBN 978-950-06-2538-8.
- ↑ Pinsky L, Ericsson RP, Schimke RN (1999). Genetic Disorders of Human Sexual Development. Oxford University Press. pp. 215–. ISBN 978-0-19-510907-8.
- 1 2 Di Lorenzo G, Autorino R, Perdonà S, De Placido S (2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- ↑ Wassersug RJ, Oliffe JL (April 2009). "The social context for psychological distress from iatrogenic gynecomastia with suggestions for its management". The Journal of Sexual Medicine. 6 (4): 989–1000. doi:10.1111/j.1743-6109.2008.01053.x. PMID 19175864.
By themselves, the LH-RH agonists do not produce much gynecomastia (ie, estimates as low as 4.4%) [13], but in conjunction with the typically prescribed antiandrogens (flutamide, bicalutamide, and nilutamide), gynecomastia is more common (49–68%) [13].
- 1 2 3 4 Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW (January 2000). "Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men". The American Journal of Surgical Pathology. 24 (1): 74–80. doi:10.1097/00000478-200001000-00009. PMID 10632490.
- 1 2 Lawrence AA (2006). "Transgender Health Concerns". In Meyer IH, Northridge ME (eds.). The Health of Sexual Minorities Public Health Perspectives on Lesbian, Gay, Bisexual and Transgender Populations. New York: Springer. pp. 473–505. doi:10.1007/978-0-387-31334-4_19. ISBN 978-0-387-28871-0.
- 1 2 Rosen PP (2009). Rosen's Breast Pathology (3 ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 31–. ISBN 978-0-7817-7137-5.
- ↑ Lorincz AM, Sukumar S (2006). "Molecular links between obesity and breast cancer". Endocrine-Related Cancer. 13 (2): 279–92. doi:10.1677/erc.1.00729. PMID 16728564.
Adipocytes make up the bulk of the human breast, with epithelial cells accounting for only approximately 10% of human breast volume.
- ↑ Howard BA, Gusterson BA (2000). "Human breast development". Journal of Mammary Gland Biology and Neoplasia. 5 (2): 119–37. doi:10.1023/A:1026487120779. PMID 11149569. S2CID 10819224.
In the stroma, there is an increase in the amount of fibrous and fatty tissue, with the adult nonlactating breast consisting of 80% or more of stroma.
- ↑ Sperling MA (10 April 2014). Pediatric Endocrinology. Elsevier Health Sciences. pp. 598–. ISBN 978-1-4557-5973-6.
Estrogen stimulates the nipples to grow, mammary terminal duct branching to progress to the stage at which ductules are formed, and fatty stromal growth to increase until it constitutes about 85% of the mass of the breast. [...] Lobulation appears around menarche, when multiple blind saccular buds form by branching of the terminal ducts. These effects are due to the presence of progesterone. [...] Full alveolar development normally only occurs during pregnancy under the influence of additional progesterone and prolactin.
- ↑ Hagisawa S, Shimura N, Arisaka O (2012). "Effect of excess estrogen on breast and external genitalia development in growth hormone deficiency". Journal of Pediatric and Adolescent Gynecology. 25 (3): e61–3. doi:10.1016/j.jpag.2011.11.005. PMID 22206682.
Estrogen stimulates growth of the nipples, progression of mammary duct branching to the stage at which ductiles are formed, and fatty stromal growth until it constitutes about 85% of the mass of the breast.
- 1 2 J. Aiman (6 December 2012). Infertility: Diagnosis and Management. Springer Science & Business Media. pp. 182–. ISBN 978-1-4613-8265-2.
- ↑ Kroemer RW (2009). The Reproductive System. Infobase Publishing. pp. 51–. ISBN 978-1-4381-3083-5.
- ↑ Fody EP, Walker EM (1985). "Effects of drugs on the male and female reproductive systems". Ann. Clin. Lab. Sci. 15 (6): 451–8. PMID 4062226.
- ↑ Liu YX (2005). "Control of spermatogenesis in primate and prospect of male contraception". Arch. Androl. 51 (2): 77–92. doi:10.1080/01485010490485768. PMID 15804862. S2CID 25411118.
- ↑ Cheng CY, Wong EW, Yan HH, Mruk DD (2010). "Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances". Mol. Cell. Endocrinol. 315 (1–2): 49–56. doi:10.1016/j.mce.2009.08.004. PMC 3516447. PMID 19682538.
- ↑ Schill W, Comhaire FH, Hargreave TB (26 August 2006). Andrology for the Clinician. Springer Science & Business Media. pp. 76–. ISBN 978-3-540-33713-3. Archived from the original on 26 May 2016.
- 1 2 Cheng C (24 October 2009). Molecular Mechanisms in Spermatogenesis. Springer Science & Business Media. pp. 258–. ISBN 978-0-387-09597-4.
- 1 2 3 Morgante E, Gradini R, Realacci M, Sale P, D'Eramo G, Perrone GA, Cardillo MR, Petrangeli E, Russo M, Di Silverio F (March 2001). "Effects of long-term treatment with the anti-androgen bicalutamide on human testis: an ultrastructural and morphometric study". Histopathology. 38 (3): 195–201. doi:10.1046/j.1365-2559.2001.01077.x. PMID 11260298. S2CID 36892099.
- 1 2 Bjerklund Johansen TE, Majak M, Nesland JM (March 1994). "Testicular histology after treatment with the new antiandrogen Casodex for carcinoma of the prostate. A preliminary report". Scand. J. Urol. Nephrol. 28 (1): 67–70. doi:10.3109/00365599409180473. PMID 8009196.
- ↑ Jones HB, Betton GR, Bowdler AL, McFarquhar RL, Middleton BJ, Lunglmayr G (1994). "Pathological and morphometric assessment of testicular parameters in patients with metastatic prostate cancer following treatment with either the antiandrogen Casodex (ZM176,334) or bilateral orchidectomy". Urol. Res. 22 (3): 191–5. doi:10.1007/BF00571849. PMID 7992465. S2CID 19540140.
- ↑ Lunglmayr G, Girsch E, Kuber W, Biegelmayr C (1988). Effect of a new non-steroidal antiandrogen (ICI 176,334) on hormone levels and testis morphology of patients with advanced prostate cancer (abstract 583). Proceedings of the 21st Congress of the International Society of Urology, October 9–14, 1988, Buenos Aires, Argentina.
- ↑ Johnson LR (14 October 2003). Essential Medical Physiology. Academic Press. pp. 731–. ISBN 978-0-08-047270-6. Archived from the original on 15 February 2017.
- ↑ Mulhall JP (21 February 2013). Fertility Preservation in Male Cancer Patients. Cambridge University Press. pp. 84–. ISBN 978-1-139-61952-3. Archived from the original on 29 April 2016.
- ↑ Iswaran TJ, Imai M, Betton GR, Siddall RA (May 1997). "An overview of animal toxicology studies with bicalutamide (ICI 176,334)". The Journal of Toxicological Sciences. 22 (2): 75–88. doi:10.2131/jts.22.2_75. PMID 9198005.
- ↑ Khursheed A, Minhas LA, Diaz WA (September 2011). "Histomorphometric study of effects of bicalutamide on spermatogenesis in male rats" (PDF). Pakistan Armed Forces Medical Journal (3). Archived (PDF) from the original on 23 June 2016.
- ↑ Khurshid A, Hamid S, Niaz WA (2014). "Effect of Bicalutamide; an Antiandrogen, on Testicular Weight in Adult Rats". Pak Armed Forces Med J. 64 (2): 204–07. ISSN 0030-9648.
- ↑ Abdulrahman AS, Mustafa IA (2019). "Impact of bicalutamide, an anti-androgen on rat testis". Zanco Journal of Pure and Applied Sciences. 31 (2). doi:10.21271/ZJPAS.31.2.12. ISSN 2218-0230.
- ↑ Chandolia RK, Weinbauer GF, Behre HM, Nieschlag E (March 1991). "Evaluation of a peripherally selective antiandrogen (Casodex) as a tool for studying the relationship between testosterone and spermatogenesis in the rat". J. Steroid Biochem. Mol. Biol. 38 (3): 367–75. doi:10.1016/0960-0760(91)90109-I. PMID 1848993. S2CID 19423860.
- ↑ Chandolia RK, Weinbauer GF, Simoni M, Behre HM, Nieschlag E (November 1991). "Comparative effects of chronic administration of the non-steroidal antiandrogens flutamide and Casodex on the reproductive system of the adult male rat". Acta Endocrinol. 125 (5): 547–55. doi:10.1530/acta.0.1250547. PMID 1759544.
- 1 2 Dohle GR, Smit M, Weber RF (November 2003). "Androgens and male fertility". World Journal of Urology. 21 (5): 341–5. doi:10.1007/s00345-003-0365-9. PMID 14566423. S2CID 29032693.
- 1 2 Basu SC (15 December 2011). Male Reproductive Dysfunction. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 323–. ISBN 978-93-5025-703-6.
- 1 2 3 4 Neumann, F.; Schenck, B. (1980). "Antiandrogens: Basic Concepts and Clinical Trials". Regulation of Male Fertility. pp. 93–106. doi:10.1007/978-94-009-8875-0_10. ISBN 978-94-009-8877-4.
- 1 2 William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 642–643. ISBN 978-0-9828280-1-4.
- 1 2 Jones CA, Reiter L, Greenblatt E (2016). "Fertility preservation in transgender patients". International Journal of Transgenderism. 17 (2): 76–82. doi:10.1080/15532739.2016.1153992. ISSN 1553-2739. S2CID 58849546.
Traditionally, patients have been advised to cryopreserve sperm prior to starting cross-sex hormone therapy as there is a potential for a decline in sperm motility with high-dose estrogen therapy over time (Lubbert et al., 1992). However, this decline in fertility due to estrogen therapy is controversial due to limited studies.
- 1 2 3 4 5 6 Payne AH, Hardy MP (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 422–431. ISBN 978-1-59745-453-7.
Estrogens are highly efficient inhibitors of the hypothalamic-hypophyseal-testicular axis (212–214). Aside from their negative feedback action at the level of the hypothalamus and pituitary, direct inhibitory effects on the testis are likely (215,216). [...] The histology of the testes [with estrogen treatment] showed disorganization of the seminiferous tubules, vacuolization and absence of lumen, and compartmentalization of spermatogenesis.
- ↑ Wakelin SH, Maibach HI, Archer CB (1 June 2002). Systemic Drug Treatment in Dermatology: A Handbook. CRC Press. pp. 32–. ISBN 978-1-84076-013-2. Archived from the original on 25 July 2014.
[Cyproterone acetate] inhibits spermatogenesis and produces reversible infertility (but is not a male contraceptive).
- 1 2 Neumann F (1994). "The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research". Exp. Clin. Endocrinol. 102 (1): 1–32. doi:10.1055/s-0029-1211261. PMID 8005205.
Spermatogenesis is also androgen-dependent and is inhibited by CPA, meaning that patients treated with high doses of CPA are sterile (Figure 23). All the effects of CPA are fully reversible.
- 1 2 Salam MA (2003). Principles & Practice of Urology: A Comprehensive Text. Universal-Publishers. pp. 684–. ISBN 978-1-58112-412-5.
Estrogens act primarily through negative feedback at the hypothalamic-pituitary level to reduce LH secretion and testicular androgen synthesis. [...] Interestingly, if the treatment with estrogens is discontinued after 3 yr. of uninterrupted exposure, serum testosterone may remain at castration levels for up to another 3 yr. This prolonged suppression is thought to result from a direct effect of estrogens on the Leydig cells.
- 1 2 3 Mast N, Lin JB, Pikuleva IA (September 2015). "Marketed Drugs Can Inhibit Cytochrome P450 27A1, a Potential New Target for Breast Cancer Adjuvant Therapy". Molecular Pharmacology. 88 (3): 428–36. doi:10.1124/mol.115.099598. PMC 4551053. PMID 26082378.
- ↑ Mast N, Zheng W, Stout CD, Pikuleva IA (February 2013). "Binding of a cyano- and fluoro-containing drug bicalutamide to cytochrome P450 46A1: unusual features and spectral response". The Journal of Biological Chemistry. 288 (7): 4613–24. doi:10.1074/jbc.M112.438754. PMC 3576067. PMID 23288837.
- 1 2 Zhu Y, Liu C, Armstrong C, Lou W, Sandher A, Gao AC (September 2015). "Antiandrogens Inhibit ABCB1 Efflux and ATPase Activity and Reverse Docetaxel Resistance in Advanced Prostate Cancer". Clinical Cancer Research. 21 (18): 4133–42. doi:10.1158/1078-0432.CCR-15-0269. PMC 4573793. PMID 25995342.
- 1 2 Fenner A (July 2015). "Prostate cancer: Antiandrogens reverse docetaxel resistance via ABCB1 inhibition". Nature Reviews. Urology. 12 (7): 361. doi:10.1038/nrurol.2015.135. PMID 26057062. S2CID 29729317.
- 1 2 Armstrong CM, Gao AC (2015). "Drug resistance in castration resistant prostate cancer: resistance mechanisms and emerging treatment strategies". American Journal of Clinical and Experimental Urology. 3 (2): 64–76. PMC 4539108. PMID 26309896.
- 1 2 3 4 5 Foster WR, Car BD, Shi H, Levesque PC, Obermeier MT, Gan J, Arezzo JC, Powlin SS, Dinchuk JE, Balog A, Salvati ME, Attar RM, Gottardis MM (April 2011). "Drug safety is a barrier to the discovery and development of new androgen receptor antagonists". The Prostate. 71 (5): 480–8. doi:10.1002/pros.21263. PMID 20878947. S2CID 24620044.
- ↑ Barrish J, Carter P, Cheng P (2010). Accounts in Drug Discovery: Case Studies in Medicinal Chemistry. Royal Society of Chemistry. pp. 127–. ISBN 978-1-84973-126-3.
- ↑ Saqib U, Savai R, Liu D, Banerjee S, Baig MS (October 2018). "Drug repositioning as an effective therapy for protease-activated receptor 2 inhibition". J. Cell. Biochem. 120 (2): 1522–1526. doi:10.1002/jcb.27334. PMID 30370939. S2CID 53099338.
- ↑ Zia MK, Siddiqui T, Ali SS, Ahsan H, Khan FH (December 2018). "Exploring the interaction of anti-androgen drug-bicalutamide with human alpha-2-macroglobulin: A biophysical investigation". Int. J. Biol. Macromol. 120 (Pt B): 2285–2292. doi:10.1016/j.ijbiomac.2018.08.117. PMID 30149080. S2CID 52094869.
- 1 2 3 4 Weber GF (22 July 2015). Molecular Therapies of Cancer. Springer. pp. 318–. ISBN 978-3-319-13278-5.
Compared to flutamide and nilutamide, bicalutamide has a 2-fold increased affinity for the Androgen Receptor, a longer half-life, and substantially reduced toxicities. Based on a more favorable safety profile relative to flutamide, bicalutamide is indicated for use in combination therapy with a Gonadotropin Releasing Hormone analog for the treatment of advanced metastatic prostate carcinoma.
- ↑ Cockshott ID, Plummer GF, Cooper KJ, Warwick MJ (October 1991). "The pharmacokinetics of Casodex in laboratory animals". Xenobiotica. 21 (10): 1347–55. doi:10.3109/00498259109043209. PMID 1796611.
- 1 2 Sharma K, Pawar GV, Giri S, Rajagopal S, Mullangi R (December 2012). "Development and validation of a highly sensitive LC-MS/MS-ESI method for the determination of bicalutamide in mouse plasma: application to a pharmacokinetic study". Biomed Chromatogr. 26 (12): 1589–95. doi:10.1002/bmc.2736. PMID 22495777.
- ↑ Kolvenbag GJ, Blackledge GR, Gotting-Smith K (January 1998). "Bicalutamide (Casodex) in the treatment of prostate cancer: history of clinical development". The Prostate. 34 (1): 61–72. doi:10.1002/(SICI)1097-0045(19980101)34:1<61::AID-PROS8>3.0.CO;2-N. PMID 9428389.
- 1 2 Tyrrell CJ, Iversen P, Tammela T, Anderson J, Björk T, Kaisary AV, Morris T (September 2006). "Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration". BJU International. 98 (3): 563–72. doi:10.1111/j.1464-410X.2006.06275.x. PMID 16771791. S2CID 41672303.
- ↑ Blackledge GR (1996). "Clinical progress with a new antiandrogen, Casodex (bicalutamide)". Eur. Urol. 29 Suppl 2 (2): 96–104. doi:10.1159/000473847. PMID 8717470.
- 1 2 3 Cantarini M, Fuhr R, Morris T (2006). "Pharmacokinetics of two novel bicalutamide formulations in healthy male volunteers". Pharmacology. 77 (4): 171–8. doi:10.1159/000094599. PMID 16847400. S2CID 35010482.
- 1 2 Chu E, DeVita Jr VT (28 December 2012). Physicians' Cancer Chemotherapy Drug Manual 2013. Jones & Bartlett Publishers. pp. 51–. ISBN 978-1-284-04039-5.
- ↑ Bunce CM, Campbell MJ (11 March 2010). Nuclear Receptors: Current Concepts and Future Challenges. Springer Science & Business Media. pp. 160, 167. ISBN 978-90-481-3303-1. Archived from the original on 10 June 2016.
- ↑ Beale C, Collins P (15 May 1996). The Cardioprotective Role of HRT: A Clinical Update. CRC Press. pp. 14–. ISBN 978-1-85070-740-0.
- 1 2 Helsen C, Van den Broeck T, Voet A, Prekovic S, Van Poppel H, Joniau S, Claessens F (August 2014). "Androgen receptor antagonists for prostate cancer therapy". Endocrine-Related Cancer. 21 (4): T105–18. doi:10.1530/ERC-13-0545. PMID 24639562.
- ↑ Furr BJ (1989). ""Casodex" (ICI 176,334)--a new, pure, peripherally-selective anti-androgen: preclinical studies". Hormone Research. 32 Suppl 1 (1): 69–76. doi:10.1159/000181315. PMID 2533159.
- ↑ Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H (June 1987). "ICI 176,334: a novel non-steroidal, peripherally selective antiandrogen". The Journal of Endocrinology. 113 (3): R7–9. doi:10.1677/joe.0.113R007. PMID 3625091.
- ↑ Soloway MS, Schellhammer PF, Smith JA, Chodak GW, Vogelzang NJ, Kennealey GT (December 1995). "Bicalutamide in the treatment of advanced prostatic carcinoma: a phase II noncomparative multicenter trial evaluating safety, efficacy and long-term endocrine effects of monotherapy". The Journal of Urology. 154 (6): 2110–4. doi:10.1016/S0022-5347(01)66709-0. PMID 7500470.
- 1 2 Gao W, Dalton JT (March 2007). "Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs)". Drug Discovery Today. 12 (5–6): 241–8. doi:10.1016/j.drudis.2007.01.003. PMC 2072879. PMID 17331889.
- ↑ Mason M (August 2006). "What implications do the tolerability profiles of antiandrogens and other commonly used prostate cancer treatments have on patient care?". Journal of Cancer Research and Clinical Oncology. 132 Suppl 1: S27–35. doi:10.1007/s00432-006-0134-4. PMID 16896883. S2CID 19685819.
- ↑ Anderson PO, Knoben JE, Troutman WG (22 August 2001). Handbook of Clinical Drug Data. Canadian Medical Association Journal. Vol. 128. McGraw Hill Professional. p. 245. ISBN 978-0-07-138942-6. PMC 1875767. PMID 20313924.
With an oral dose of 50 mg/day, bicalutamide attains a peak serum level of 8.9 mg/L (21 μmol/L) 31 hr after a dose at steady state. CI of (R)-bicalutamide is 0.32 L/hr. The active (R)-enantiomer of bicalutamide is oxidized to an inactive metabolite, which, like the inactive (S)-enantiomer, is glucuronidated and cleared rapidly by elimination in the urine and feces.165
- ↑ Cockshott ID (2004). "Bicalutamide: clinical pharmacokinetics and metabolism". Clinical Pharmacokinetics. 43 (13): 855–878. doi:10.2165/00003088-200443130-00003. PMID 15509184.
These data indicate that direct glucuronidation is the main metabolic pathway for the rapidly cleared (S)-bicalutamide, whereas hydroxylation followed by glucuronidation is a major metabolic pathway for the slowly cleared (R)-bicalutamide.
- ↑ Grosse L, Campeau AS, Caron S, Morin FA, Meunier K, Trottier J, Caron P, Verreault M, Barbier O (August 2013). "Enantiomer selective glucuronidation of the non-steroidal pure anti-androgen bicalutamide by human liver and kidney: role of the human UDP-glucuronosyltransferase (UGT)1A9 enzyme". Basic & Clinical Pharmacology & Toxicology. 113 (2): 92–102. doi:10.1111/bcpt.12071. PMC 3815647. PMID 23527766.
- 1 2 McKillop, D.; Boyle, G. W.; Cockshott, I. D.; Jones, D. C.; Phillips, P. J.; Yates, R. A. (1993). "Metabolism and enantioselective pharmacokinetics of Casodex in man". Xenobiotica. 23 (11): 1241–1253. doi:10.3109/00498259309059435. ISSN 0049-8254. PMID 8310708.
- 1 2 Cockshott, I.D.; Cooper, K.J.; Sweetmore, D.S.; Blacklock, N.J.; Denis, L. (1990). "The Pharmacokinetics of Casodex in Prostate Cancer Patients after Single and during Multiple Dosing". European Urology. 18 (3): 10–17. doi:10.1159/000463972. ISSN 0302-2838. PMID 2094606.
- 1 2 Tyrrell CJ, Iversen P, Tammela T, Anderson J, Björk T, Kaisary AV, Morris T (September 2006). "Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration". BJU International. 98 (3): 563–72. doi:10.1111/j.1464-410X.2006.06275.x. PMID 16771791. S2CID 41672303.
- 1 2 3 Cockshott ID (2004). "Bicalutamide: clinical pharmacokinetics and metabolism". Clinical Pharmacokinetics. 43 (13): 855–878. doi:10.2165/00003088-200443130-00003. PMID 15509184.
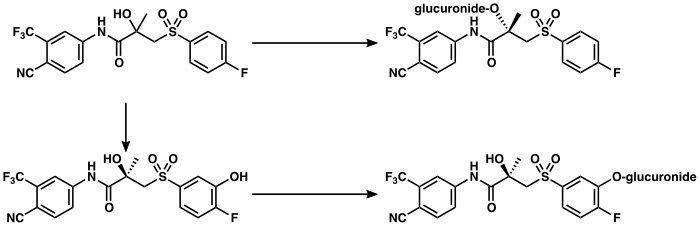
-Bicalutamide_levels_with_a_single_5_to_80_mg_oral_dose_of_bicalutamide_in_men.png.webp)