Bisphenol A
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′-(Propane-2,2-diyl)diphenol | |
| Other names
BPA, p,p-Isopropylidenebisphenol, 2,2-Bis(4-hydroxyphenyl)propane | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.133 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2924 2430 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H16O2 |
| Molar mass | 228.291 g·mol−1 |
| Appearance | White solid |
| Density | 1.20 g/cm3 |
| Melting point | 158 to 159 °C (316 to 318 °F; 431 to 432 K) |
| Boiling point | 360 °C (680 °F; 633 K) |
Solubility in water |
120–300 ppm (21.5 °C) |
| Vapor pressure | 5×10−6 Pa (25 °C)[1] |
| Hazards | |
| GHS labelling: | |
Pictograms |
   |
Signal word |
Danger |
Hazard statements |
H317, H318, H335, H360 |
Precautionary statements |
P201, P202, P261, P271, P272, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P312, P321, P333+P313, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | |
| Flash point | 227 °C (441 °F; 500 K) |
Autoignition temperature |
600 °C (1,112 °F; 873 K) |
| Related compounds | |
Related phenols |
Bisphenol S |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
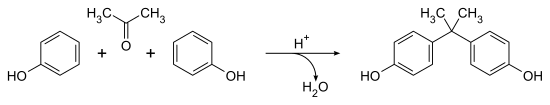
Bisphenol A (BPA) is a chemical compound and one of the simplest and best known bisphenols. It is produced by the condensation of phenol and acetone, with an estimated 4 million tonnes of produced worldwide in 2015.[2] It is a colourless solid which is soluble in organic solvents, but poorly soluble in water (0.344 wt % at 83 °C).[3]
BPA and its derivatives have many uses, most of which are centred around plastics. Its largest single application is as a co-monomer in the production of polycarbonates and, to a much lesser extent, polysulfones. Its epoxide derivative BADGE (also called DGEBA) is the starting material for most epoxy resins. Low levels of unpolymerised BPA and BADGE are also used in PVC plastisols,[4][5] as an auxiliary antioxidant and acid scavenger respectively. A common, if minor, use is as a stabiliser in thermal paper.[6] It is not a plasticizer,[7] although it is often wrongly labelled as such.
BPA is a xenoestrogen, exhibiting estrogen-mimicking, hormone-like properties.[8] Although the effect is very weak, the pervasiveness of BPA-containing materials raises concerns. Many of these materials are non-obvious but commonly encountered;[9] such as coatings for the inside of food cans,[10] clothing,[11] shop receipts[6] and dental fillings.[12] Since 2008, several governments have investigated its safety, which prompted some retailers to withdraw polycarbonate products. Since then, BPA-free plastics have been manufactured using alternative bisphenols such as bisphenol S and bisphenol F, but there is controversy around whether these are actually safer.[13]
History
Bisphenol A was reported in 1891 by the Russian chemist Aleksandr Dianin.[14]
In 1934 workers at I.G. Farbenindustrie reported the coupling of BPA and epichlorohydrin. Over the following decade, coatings and resins derived from similar materials were described by workers at the companies of DeTrey Freres in Switzerland and DeVoe and Raynolds in the US. This early work underpinned the development of epoxy resins, which in turn motivated production of BPA.[15] The utilization of BPA further expanded with discoveries at Bayer and General Electric on polycarbonate plastics. These plastics first appeared in 1958, being produced by Mobay and General Electric, and Bayer.[16]
In terms of the endocrine disruption controversy, the British biochemist Edward Charles Dodds tested BPA as an artificial estrogen in the early 1930s. He found BPA to be 1 / 37,000 as effective as estradiol.[17][18][19] Dodds eventually developed a structurally similar compound, diethylstilbestrol (DES), which was used as a synthetic estrogen drug in women and animals until it was banned due to its risk of causing cancer; the ban on use of DES in humans came in 1971 and in animals, in 1979.[17] BPA was never used as a drug.[17]
Production
The synthesis of BPA still follows Dianin's general method, with the fundamentals having changed little in 130 years. The condensation of acetone (hence the suffix A in the name)[20] with two equivalents of phenol is catalyzed by a strong acid, such as concentrated hydrochloric acid, sulfuric acid, or a solid acid resin such as polystyrene sulfonate.[21] An excess of phenol is used to ensure full condensation and to limit the formation of by-products, such as Dianin's compound. Large amounts of both starting materials are available from the cumene process,[3] making it fairly cheap to produce. Global production in 2022 is expected to reach 10 million tonnes.[22]
The reaction is strongly para selective but minor amounts of the ortho-para (up 3%) and ortho-ortho isomers are also produced, along with several other minor by-products.[23] These are not always removed and are known impurities in commercial samples of BPA.[24][23]
Uses and applications

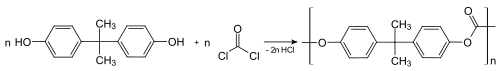
Polycarbonates
Between about 65-70% of all bisphenol A is used to make polycarbonate plastics,[25][26] which can consists of nearly 90% BPA by mass. Polymerisation is achieved by a reaction with phosgene, conducted under biphasic conditions; the hydrochloric acid is scavenged with aqueous base.[27] This process converts the individual molecules of BPA into large polymer chains, effectively trapping them.
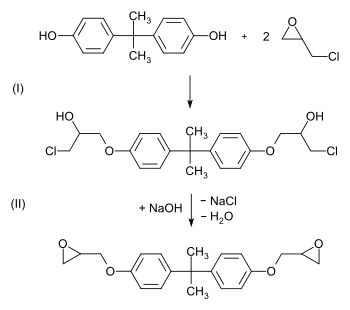
Epoxy and vinyl ester resins
About 25-30% of all BPA is used in the manufacture of epoxy resins and vinyl ester resins.[25][26] For epoxy resin it is first converted to its diglycide ether (usually abbreviated BADGE or DGEBA).[28][29] This is achieved by a reaction with epichlorohydrin under basic conditions.
Some of this is further reacted with methacrylic acid to form bis-GMA, which is used to make vinyl ester resins. Alternatively, and to a much lesser extent, BPA may be ethoxylated and then converted to its diacrylate and dimethacrylate derivatives. These may be incorporated at low levels in vinyl ester resins to change their physical properties.[30]
Minor uses
The remaining 5% of BPA is used in a wide range of applications, many of which are also to do with plastic.[31] BPA is a major component of several high-performance plastics, the production of these is low compared to other plastics but still equals several thousand tons a year. Comparatively minor amounts of BPA are also used as additives or modifiers in some commodity plastics. These materials are much more common but their BPA content will be minimal.
Plastics
- As a major component
- Polycyanurates can be produced from BPA by way of its cyanate ester.[31] This is itself formed by a reaction between BPA and cyanogen bromide. Examples include BT-Epoxy, which is one of a number of resins used the production of printed circuit boards.
- Polyetherimides can be produced from BPA.[32] These plastics have exceptional resistance to mechanical, thermal and chemical damage. They find use in medical devices and other high performance instrumentation.
- Polybenzoxazines may be produced from a number of biphenols, including BPA.[33][34]
- Polysulfones can be produced from BPA and bis(4-chlorophenyl) sulfone forming poly(bisphenol-A sulfone) (PSF). It is used as a high performance alternative to polycarbonate.[31]
- Bisphenol-A formaldehyde resins are a subset of phenol formaldehyde resins. They are used in the production of high-pressure laminates[31]
- As a minor component
- Polyurethane foams, and particularly memory foams, sometimes use the diacrylate and dimethacrylate derivatives of ethoxylated BPA as chain extenders.[35]
- PVC can contain BPA and its derivatives through multiple routes. BPA is sometimes used as an antioxidant in phthalates,[36] which are extensively used as plasticizers for PVC. BPA has also been used as an antioxidant to protect sensitive PVC heat stabilizers. Historically between 5 and 10% by weight of BPA was included in barium cadmium types, and from 5 to 10% of BHT was included in nontoxic calcium zinc solids. BPA diglycidyl ether (BADGE) is used as an acid scavenger, particularly in PVC dispersions, such as organosols or plastisols, which are used as coatings for the inside of food cans, as well as embossed clothes designs produced using heat transfer vinyl or screen printing machines.[37]
- Bromination of BPA froms tetrabromobisphenol A (TBBPA), which is used as a flame retardant in plastics.[38] TBBPA is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistant polycarbonates by replacing some bisphenol A. A lower grade of TBBPA is used to prepare epoxy resins, used in printed circuit boards. Its use is diminishing due to restrictions on brominated flame retardants.
Other applications
- BPA finds use as an antioxidant in several fields, partiually in brake fluids.[39]
- BPA is used as a developering agent in thermal paper (shop receipts).[40][41] Recycled paper products can also contain BPA as a result.
- Ethoxylated BPA finds minor use as a 'levelling agent' in tin electroplating.
- Several drug candidates have also been developed from bisphenol A, including Ralaniten, Ralaniten acetate, and EPI-001.
BPA substitutes
Concerns about the health effects of BPA have led many manufacturers to replace BPA with substitutes such as bisphenol S (BPS) and diphenyl sulfone. However, health concerns have been raised about these substitutes as well.[42] In the similar compound Bisphenol F, the F signifies formaldehyde Numerous ketones undergo analogous condensation reactions.[3]
| Structural formula | Name | CAS | Reactants | |
|---|---|---|---|---|
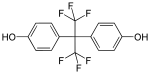 | Bisphenol AF | 1478-61-1 | Phenol | Hexafluoroacetone |
| Bisphenol F | 620-92-8 | Phenol | Formaldehyde | |
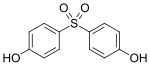 | Bisphenol S | 80-09-1 | Phenol | Sulfur trioxide |
 | Bisphenol Z | 843-55-0 | Phenol | Cyclohexanone |
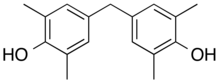 | Tetramethyl bisphenol F | 5384-21-4 | 2,6-xylenol | Formaldehyde |
Health effects
BPA's ability to mimic the effects of natural estrogen derive from the similarity of phenol groups on both BPA and estradiol, which enable this synthetic molecule to trigger estrogenic pathways in the body.[45] Typically phenol-containing molecules similar to BPA are known to exert weak estrogenic activities, thus it is also considered an endocrine disruptor (ED) and estrogenic chemical.[46] Xenoestrogens is another category the chemical BPA fits under because of its capability to interrupt the network that regulates the signals which control the reproductive development in humans and animals.[47]
BPA has been found to bind to both of the nuclear estrogen receptors (ERs), ERα and ERβ. It is 1000- to 2000-fold less potent than estradiol. BPA can both mimic the action of estrogen and antagonize estrogen, indicating that it is a selective estrogen receptor modulator (SERM) or partial agonist of the ER. At high concentrations, BPA also binds to and acts as an antagonist of the androgen receptor (AR). In addition to receptor binding, the compound has been found to affect Leydig cell steroidogenesis, including affecting 17α-hydroxylase/17,20 lyase and aromatase expression and interfering with LH receptor-ligand binding.
In 1997, adverse effects of low-dose BPA exposure in laboratory animals were first proposed.[48] Some studies have found that BPA increases anxiety in rats.[49][50] Modern studies began finding possible connections to health issues caused by exposure to BPA during pregnancy and during development. As of 2014, research and debates are ongoing as to whether BPA should be banned or not.
A 2007 study investigated the interaction between bisphenol A's and estrogen-related receptor γ (ERR-γ). This orphan receptor (endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (dissociation constant = 5.5 nM), but only weakly to the ER.[51] BPA binding to ERR-γ preserves its basal constitutive activity.[51] It can also protect it from deactivation from the SERM 4-hydroxytamoxifen (afimoxifene).[51] This may be the mechanism by which BPA acts as a xenoestrogen.[51] Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. BPA has also been found to act as an agonist of the GPER (GPR30).[52]
According to the European Food Safety Authority "BPA poses no health risk to consumers of any age group (including unborn children, infants and adolescents) at current exposure levels".[53] But in 2017 the European Chemicals Agency concluded that BPA should be listed as a substance of very high concern due to its properties as an endocrine disruptor.[54]
In 2012, the United States' Food and Drug Administration (FDA) banned the use of BPA in baby bottles.[55]
The U.S. Environmental Protection Agency (EPA) holds the position that BPA is not a health concern. In 2011, Andrew Wadge, the chief scientist of the United Kingdom's Food Standards Agency, commented on a 2011 U.S. study on dietary exposure of adult humans to BPA,[56] saying, "This corroborates other independent studies and adds to the evidence that BPA is rapidly absorbed, detoxified, and eliminated from humans – therefore is not a health concern."[57]
The Endocrine Society said in 2015 that the results of ongoing laboratory research gave grounds for concern about the potential hazards of endocrine-disrupting chemicals – including BPA – in the environment, and that on the basis of the precautionary principle these substances should continue to be assessed and tightly regulated.[58] A 2016 review of the literature said that the potential harms caused by BPA were a topic of scientific debate and that further investigation was a priority because of the association between BPA exposure and adverse human health effects including reproductive and developmental effects and metabolic disease.[59]
In July 2019, the European Union upheld a decision by the European Chemicals Agency to list BPA as a substance of very high concern, the first step in the procedure for restrictions of its use. The decision is based on concerns about BPA's toxicity for human reproduction.[60]
Environmental effects
In 2010, the U.S. Environmental Protection Agency reported that over one million pounds of BPA are released into the environment annually.[61] BPA can be released into the environment by both pre-consumer and post-consumer leaching. Common routes of introduction from the pre-consumer perspective into the environment are directly from plastics, coat and staining manufacturers, foundries who use BPA in casting sand, or transport of BPA and BPA-containing products.[62][63] Post-consumer BPA waste comes from effluent discharge from municipal wastewater treatment plants, irrigation pipes used in agriculture, ocean-borne plastic trash, indirect leaching from plastic, paper, and metal waste in landfills, and paper or material recycling companies.[62][63][64] Despite a rapid soil and water half-life of 4.5 days, and an air half-life of less than one day, BPA's ubiquity makes it an important pollutant. BPA has a low rate of evaporation from water and soil, which presents issues, despite its biodegradability and low concern for bio-accumulation. BPA has low volatility in the atmosphere and a low vapor pressure between 5.00 and 5.32 Pascals. Aqueous solutions of BPA absorbs at wavelengths greater than 250 nm.[65]
BPA interferes with nitrogen fixation at the roots of leguminous plants associated with the bacterial symbiont Sinorhizobium meliloti.[66] BPA affects soybean seedlings with respect to root growth, nitrate production, ammonium production, and the activities of nitrate reductase and nitrite reductase. At low doses of BPA, the growth of roots were improved, the amount of nitrate in roots increased, the amount of ammonium in roots decreased, and the nitrate and nitrite reductase activities remained unchanged. However, at considerably higher concentrations of BPA, the opposite effects were seen for all but an increase in nitrate concentration and a decrease in nitrite and nitrate reductase activities.[67] Nitrogen is both a plant nutritional substance, but also the basis of growth and development in plants.
A 2005 study conducted in the United States had found that 91–98% of BPA may be removed from water during treatment at municipal water treatment plants.[68] Nevertheless, a 2009 meta-analysis of BPA in the surface water system showed BPA present in surface water and sediment in the U.S. and Europe.[69] According to Environment Canada in 2011, "BPA can currently be found in municipal wastewater. … initial assessment shows that at low levels, bisphenol A can harm fish and organisms over time."[70]
BPA affects growth, reproduction, and development in aquatic organisms. Among freshwater organisms, fish appear to be the most sensitive species. Evidence of endocrine-related effects in fish, aquatic invertebrates, amphibians, and reptiles has been reported at environmentally relevant exposure levels lower than those required for acute toxicity. There is a widespread variation in reported values for endocrine-related effects, but many fall in the range of 1 μg/L to 1 mg/L.[71]
A 2009 review by the Royal Society, with a focus on aquatic and terrestrial annelids, molluscs, crustaceans, insects, fish and amphibians concluded that BPA affects reproduction in all studied animal groups, impairs development in crustaceans and amphibians and induces genetic aberrations.[72]
Toxicity
BPA exhibits very low acute toxicity as indicated by its LD50 of 4 g/kg (mouse). In those mice, weight gain was reduced and exhibited estrogen-like properties. Reports indicate that it is a minor skin irritator as well, although less so than phenol.[3]
The FDA's National Center for Toxicology Research conducted its own research studies. In rodent studies, the amount of BPA passed from the mother to the unborn offspring after oral administration was found to be insignificant. The BPA administration dose for the rodents was 100-1000 times higher than human exposure.[73]
Identification in plastics
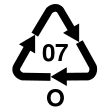
In the U.S., plastic packaging is split into seven broad classes for identification purposes by a resin identification code. As of 2014 there are no BPA labeling requirements for plastics in the U.S. "In general, plastics that are marked with Resin Identification Codes 1, 2, 4, 5, and 6 are very unlikely to contain BPA. Some, but not all, plastics that are marked with the Resin Identification Code 7 may be made with BPA."[74] Type 7 is the catch-all "other" class, and some type 7 plastics, such as polycarbonate (sometimes identified with the letters "PC" near the recycling symbol) and epoxy resins, are made from bisphenol A monomer.[3][75] Type 3 (PVC) may contain bisphenol A as an antioxidant (not a plasticizer) in "flexible PVC" softened by plasticizers,[3] but not rigid PVC such as pipe, window frames, and siding.
Legislation
The U.S. Food and Drug Administration (FDA) has ended its authorization of the use of BPA in baby bottles and infant formula packaging, based on market abandonment, not safety.[76] The European Union[77] and Canada have banned BPA use in baby bottles.
Currently in the United States, there are 12 states, in addition to Washington, D.C., that have restrictions in place against BPA. These states include California, Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, Nevada, New York, Vermont, Washington, and Wisconsin. Each state's restrictions differ slightly, but all restrict the use of BPA in some way.[78]
The following are some examples of legislation in place in these states:
- California - Assembly Bill 1319 (2011) prohibits the manufacture and sale of bottles and cups with BPA at detectable levels above 0.1 parts per billion if these items are meant to be used by children 3 years old or younger. It requires manufacturers to use alternative non-toxic materials that are not categorized as carcinogenic or reproductive toxicants.[79]
- Delaware - Senate Bill 70 (2011) prohibits the sale of bottles and cups containing BPA if the items are designed to be used by children 4 years old or younger.[80]
- Illinois - Senate Bill 2950 (2011) prohibits the sale of empty containers like bottles or cups designed to store food and beverage for children that contain BPA.[81]
- Maine - House Bill 330 (2011) helped process BPA to be recognized as a priority chemical under Title 38, §1691, Maine's law on toxic chemicals in products designed to be used by children. BPA was formally designated as a substance that must pass through certain reporting requirements for manufactureres of products that contain BPA, as well as authorize the prohibition of sale of products that are reported to contain BPA.[82]
See also
- Tetramethyl bisphenol F
- Bisphenol S
- p-tert-Butylphenol
References
- ↑ "Chemical Fact Sheet – Cas #80057 CASRN 80-05-7". speclab.com. 1 April 2012. Archived from the original on 12 February 2012. Retrieved 14 June 2012.
- ↑ "Archived copy". Archived from the original on 19 March 2017. Retrieved 24 January 2017.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 3 4 5 6 Fiege H, Voges HW, Hamamoto T, Umemura S, Iwata T, Miki H, Fujita Y, Buysch HJ, Garbe D, Paulus W (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ Shah, Ashok C.; Poledna, David J. (September 2003). "Review of PVC dispersion and blending resin products". Journal of Vinyl and Additive Technology. 9 (3): 146–154. doi:10.1002/vnl.10076.
- ↑ Shah, Ashok C.; Poledna, David J. (September 2002). "Review of specialty PVC resins". Journal of Vinyl and Additive Technology. 8 (3): 214–221. doi:10.1002/vnl.10365.
- 1 2 Björnsdotter, Maria K.; de Boer, Jacob; Ballesteros-Gómez, Ana (September 2017). "Bisphenol A and replacements in thermal paper: A review". Chemosphere. 182: 691–706. Bibcode:2017Chmsp.182..691B. doi:10.1016/j.chemosphere.2017.05.070. PMID 28528315.
- ↑ Cadogan, David F.; Howick, Christopher J. (2000). "Plasticizers". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a20_439. ISBN 3527306730.
- ↑ Egan, Michael (2013). "Sarah A. Vogel. Is It Safe? BPA and the Struggle to Define the Safety of Chemicals". Isis. Berkeley: University of California Press. 105 (1): 254. doi:10.1086/676809. ISSN 0021-1753.
- ↑ Geens, Tinne; Aerts, Dominique; Berthot, Carl; Bourguignon, Jean-Pierre; Goeyens, Leo; Lecomte, Philippe; Maghuin-Rogister, Guy; Pironnet, Anne-Madeleine; Pussemier, Luc; Scippo, Marie-Louise; Van Loco, Joris; Covaci, Adrian (October 2012). "A review of dietary and non-dietary exposure to bisphenol-A". Food and Chemical Toxicology. 50 (10): 3725–3740. doi:10.1016/j.fct.2012.07.059. PMID 22889897.
- ↑ Noonan, Gregory O.; Ackerman, Luke K.; Begley, Timothy H. (13 July 2011). "Concentration of Bisphenol A in Highly Consumed Canned Foods on the U.S. Market". Journal of Agricultural and Food Chemistry. 59 (13): 7178–7185. doi:10.1021/jf201076f. PMID 21598963.
- ↑ Xue, Jingchuan; Liu, Wenbin; Kannan, Kurunthachalam (2 May 2017). "Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing". Environmental Science & Technology. 51 (9): 5279–5286. Bibcode:2017EnST...51.5279X. doi:10.1021/acs.est.7b00701. PMID 28368574.
- ↑ Ahovuo-Saloranta, Anneli; Forss, Helena; Walsh, Tanya; Nordblad, Anne; Mäkelä, Marjukka; Worthington, Helen V. (31 July 2017). "Pit and fissure sealants for preventing dental decay in permanent teeth". The Cochrane Database of Systematic Reviews. 7: CD001830. doi:10.1002/14651858.CD001830.pub5. ISSN 1469-493X. PMC 6483295. PMID 28759120.
- ↑ Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J (February 2020). "Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review". Nutrients. 12 (2): 532. doi:10.3390/nu12020532. PMC 7071457. PMID 32092919.
- ↑ See:
- А. Дианина (1891) "О продуктахъ конденсацiи кетоновъ съ фенолами" (On condensation products of ketones with phenols), Журнал Русского физико-химического общества (Journal of the Russian Physical Chemistry Society), 23 : 488-517, 523–546, 601–611; see especially pages 491-493 ("Диметилдифенолметань" (dimethyldiphenolmethane)).
- Reprinted in condensed form in: A. Dianin (1892) "Condensationsproducte aus Ketonen und Phenolen" (Condensation products of ketones and phenols), Berichte der Deutschen chemischen Gesellschaft zu Berlin, 25, part 3 : 334-337.
- ↑ Pham HQ, Marks MJ (2012). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_547.pub2.
- ↑ Volker Serini (2000). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_207.
- 1 2 3 Vogel SA (2009). "The Politics of Plastics: The Making and Unmaking of Bisphenol A "Safety". Am J Public Health. 99 (S3): S559–S566. doi:10.2105/AJPH.2008.159228. PMC 2774166. PMID 19890158.
- ↑ Dodds EC, Lawson W (1936). "Synthetic Œstrogenic Agents without the Phenanthrene Nucleus". Nature. 137 (3476): 996. Bibcode:1936Natur.137..996D. doi:10.1038/137996a0. S2CID 4171635.
- ↑ Dodds EC, Lawson W (1938). "Molecular Structure in Relation to Oestrogenic Activity. Compounds without a Phenanthrene Nucleus". Proceedings of the Royal Society of London B: Biological Sciences. 125 (839): 222–232. Bibcode:1938RSPSB.125..222D. doi:10.1098/rspb.1938.0023.
- ↑ Uglea CV, Negulescu II (1991). Synthesis and Characterization of Oligomers. CRC Press. p. 103. ISBN 978-0-8493-4954-6.
- ↑ de Angelis, A.; Ingallina, P.; Perego, C. (March 2004). "Solid Acid Catalysts for Industrial Condensations of Ketones and Aldehydes with Aromatics". Industrial & Engineering Chemistry Research. 43 (5): 1169–1178. doi:10.1021/ie030429+.
- ↑ Abraham, Anna; Chakraborty, Paromita (25 June 2020). "A review on sources and health impacts of bisphenol A". Reviews on Environmental Health. 35 (2): 201–210. doi:10.1515/reveh-2019-0034. PMID 31743105. S2CID 208186123.
- 1 2 Terasaki, Masanori; Nomachi, Makoto; Edmonds, John S; Morita, Masatoshi (May 2004). "Impurities in industrial grade 4,4′-isopropylidene diphenol (bisphenol A): possible implications for estrogenic activity". Chemosphere. 55 (6): 927–931. Bibcode:2004Chmsp..55..927T. doi:10.1016/j.chemosphere.2003.11.063. PMID 15041297.
- ↑ Pahigian, Jamie M.; Zuo, Yuegang (September 2018). "Occurrence, endocrine-related bioeffects and fate of bisphenol A chemical degradation intermediates and impurities: A review". Chemosphere. 207: 469–480. Bibcode:2018Chmsp.207..469P. doi:10.1016/j.chemosphere.2018.05.117. PMID 29807346. S2CID 44172964.
- 1 2 European Commission. Joint Research Centre. Institute for Health Consumer Protection (2010). Updated European Union risk assessment report : 4,4'-isopropylidenediphenol (bisphenol-A) : environment addendum of February 2008. Publications Office. p. 6. doi:10.2788/40195. ISBN 9789279175411.
- 1 2 Vasiljevic, Tijana; Harner, Tom (1 October 2021). "Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels". Science of the Total Environment. 789: 148013. Bibcode:2021ScTEn.789n8013V. doi:10.1016/j.scitotenv.2021.148013. PMID 34323825.
- ↑ Serini, Volker (2000). "Polycarbonates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_207.
- ↑ Ng, Feifei; Couture, Guillaume; Philippe, Coralie; Boutevin, Bernard; Caillol, Sylvain (18 January 2017). "Bio-Based Aromatic Epoxy Monomers for Thermoset Materials". Molecules. 22 (1): 149. doi:10.3390/molecules22010149. PMC 6155700. PMID 28106795.
- ↑ Kroschwitz, Jacqueline I. (1998). Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 5 (5 ed.). p. 8. ISBN 978-0-471-52695-7.
- ↑ Gonçalves, Flávia; Kawano, Yoshio; Pfeifer, Carmem; Stansbury, Jeffrey W.; Braga, Roberto R. (August 2009). "Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites". European Journal of Oral Sciences. 117 (4): 442–446. doi:10.1111/j.1600-0722.2009.00636.x. PMID 19627357.
- 1 2 3 4 Geens, Tinne; Goeyens, Leo; Covaci, Adrian (September 2011). "Are potential sources for human exposure to bisphenol-A overlooked?". International Journal of Hygiene and Environmental Health. 214 (5): 339–347. doi:10.1016/j.ijheh.2011.04.005. PMID 21570349.
- ↑ Takekoshi, T.; Kochanowski, J. E.; Manello, J. S.; Webber, M. J. (June 1985). "Polyetherimides. I. Preparation of dianhydrides containing aromatic ether groups". Journal of Polymer Science: Polymer Chemistry Edition. 23 (6): 1759–1769. Bibcode:1985JPoSA..23.1759T. doi:10.1002/pol.1985.170230616.
- ↑ Vijayakumar, C.T.; Shamim Rishwana, S.; Surender, R.; David Mathan, N.; Vinayagamoorthi, S.; Alam, S. (2 January 2014). "Structurally diverse benzoxazines: synthesis, polymerization, and thermal stability". Designed Monomers and Polymers. 17 (1): 47–57. doi:10.1080/15685551.2013.797216. S2CID 94255723.
- ↑ Ghosh, N.N.; Kiskan, B.; Yagci, Y. (November 2007). "Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties". Progress in Polymer Science. 32 (11): 1344–1391. doi:10.1016/j.progpolymsci.2007.07.002.
- ↑ Laza, José Manuel; Veloso, Antonio; Vilas, José Luis (10 January 2021). "Tailoring new bisphenol a ethoxylated shape memory polyurethanes". Journal of Applied Polymer Science. 138 (2): 49660. doi:10.1002/app.49660. S2CID 224955435.
- ↑ "European Union Summary Risk Assessment Report - Bis (2-ethylhexyl) Phthalate (DEHP)". Joint Research Centre (JRC) Publications Repository. European Commission. ISSN 1018-5593. Retrieved 24 November 2021.

- ↑ Xue, Jingchuan; Liu, Wenbin; Kannan, Kurunthachalam (2 May 2017). "Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing". Environmental Science & Technology. 51 (9): 5279–5286. Bibcode:2017EnST...51.5279X. doi:10.1021/acs.est.7b00701. PMID 28368574.
- ↑ Dagani MJ, Barda HJ, Benya TJ, Sanders DC. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
- ↑ Lamprea, Katerine; Bressy, Adèle; Mirande-Bret, Cécile; Caupos, Emilie; Gromaire, Marie-Christine (August 2018). "Alkylphenol and bisphenol A contamination of urban runoff: an evaluation of the emission potentials of various construction materials and automotive supplies". Environmental Science and Pollution Research. 25 (22): 21887–21900. doi:10.1007/s11356-018-2272-z. PMID 29796891. S2CID 44140721.
- ↑ Björnsdotter, Maria K.; de Boer, Jacob; Ballesteros-Gómez, Ana (September 2017). "Bisphenol A and replacements in thermal paper: A review". Chemosphere. 182: 691–706. Bibcode:2017Chmsp.182..691B. doi:10.1016/j.chemosphere.2017.05.070. PMID 28528315.
- ↑ Liao, Chunyang; Kannan, Kurunthachalam (1 November 2011). "Widespread Occurrence of Bisphenol A in Paper and Paper Products: Implications for Human Exposure". Environmental Science & Technology. 45 (21): 9372–9379. Bibcode:2011EnST...45.9372L. doi:10.1021/es202507f. PMID 21939283.
- ↑ "BPA substitutes may be just as bad as the popular consumer plastic". 13 September 2018.
- ↑ Heather Caliendo for PlasticsToday – Packaging Digest, 20 June 2012 History of BPA Archived 12 June 2013 at the Wayback Machine
- ↑ Walsh B (1 April 2010). "The Perils of Plastic – Environmental Toxins". Time. Archived from the original on 4 April 2010. Retrieved 2 July 2010.
- ↑ Kwon JH, Katz LE, Liljestrand HM (2007). "Modeling Binding Equilibrium in a Competitive Estrogen Receptor Binding Assay". Chemosphere. 69 (7): 1025–1031. Bibcode:2007Chmsp..69.1025K. doi:10.1016/j.chemosphere.2007.04.047. PMID 17559906.
- ↑ Ahmed, R. A. M. (2014). "Effect of Prenatal Exposure to Bisphenol A on the Vagina of Albino Rats: immunohistochemical and ultrastructural study". Folia Morphologica. 73 (4): 399–408. doi:10.5603/FM.2014.0061. PMID 25448896.
- ↑ Ramos, J.G. (2003). "Bisphenol A induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats". Endocrinology. 144 (7): 3206–3215. doi:10.1210/en.2002-0198. PMID 12810577.
- ↑ Erickson BE (2 June 2008). "Bisphenol A under scrutiny". Chemical and Engineering News. 86 (22): 36–39. doi:10.1021/cen-v086n022.p036.
- ↑ Hicks, Kimani D.; Sullivan, Alana W.; Cao, Jinyan; Sluzas, Emily; Rebuli, Meghan; Patisaul, Heather B. (August 2016). "Interaction of Bisphenol A (BPA) and soy phytoestrogens on sexually dimorphic sociosexual behaviors in male and female rats". Hormones and Behavior. 84: 121–126. doi:10.1016/j.yhbeh.2016.06.010. ISSN 0018-506X. PMC 4996731. PMID 27373758.
- ↑ Shipman, Matt (7 September 2012). "Study Finds How BPA Affects Gene Expression, Anxiety; Soy Mitigates Effects". NC State News. Retrieved 4 February 2021.
- 1 2 3 4 Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y (October 2007). "Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma". J. Biochem. 142 (4): 517–24. doi:10.1093/jb/mvm158. PMID 17761695.
- ↑ Prossnitz ER, Barton M (May 2014). "Estrogen biology: new insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ↑ "Bisphenol A". European Food Safety Authority. 2015. Lay summary.
{{cite web}}: Cite uses deprecated parameter|lay-url=(help) - ↑ "MSC unanimously agrees that Bisphenol A is an endocrine disruptor - All news - ECHA". echa.europa.eu. Retrieved 19 June 2017.
- ↑ Mirmira P, Evans-Molina C (July 2014). "Bisphenol A, obesity, and type 2 diabetes mellitus: genuine concern or unnecessary preoccupation?". Translational Research (Review). 164 (1): 13–21. doi:10.1016/j.trsl.2014.03.003. hdl:1805/8373. PMC 4058392. PMID 24686036.
- ↑ Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham MK (September 2011). "Twenty-four-hour human urine and serum profiles of bisphenol A during high-dietary exposure". Toxicological Sciences. 123 (1): 48–57. doi:10.1093/toxsci/kfr160. PMID 21705716.
- ↑ Wage, Andrew (27 July 2011). "Small pond, same big issues". FSA. Archived from the original on 10 September 2011. Retrieved 3 August 2011.
- ↑ Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT (2015). "Executive Summary to EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals". Endocr. Rev. 36 (6): 593–602. doi:10.1210/er.2015-1093. PMC 4702495. PMID 26414233.
- ↑ Giulivo M, Lopez de Alda M, Capri E, Barceló D (2016). "Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review". Environ. Res. (Review). 151: 251–264. Bibcode:2016ER....151..251G. doi:10.1016/j.envres.2016.07.011. PMID 27504873.
- ↑ Fisher, Douglas (12 July 2019). "EU court confirms BPA as substance of 'very high concern'". Environmental Health News. Retrieved 21 July 2020.
- ↑ Erler C, Novak J (October 2010). "Bisphenol a exposure: human risk and health policy". Journal of Pediatric Nursing. 25 (5): 400–7. doi:10.1016/j.pedn.2009.05.006. PMID 20816563.
- 1 2 Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW (29 July 2015). "Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation". Dose-Response. 13 (3): 1559325815598308. doi:10.1177/1559325815598308. PMC 4674187. PMID 26674671.
- 1 2 EPA (26 July 2011). "Testing of Bisphenol A, Advance notice of proposed rulemaking (ANPRM)". Federal Register /Vol. 76, No. 143 / Proposed Rules. Federal Register. Retrieved 8 May 2017.
- ↑ "Plastic Breaks Down in Ocean, After All -- And Fast". news.nationalgeographic.com. 20 August 2009. Retrieved 27 November 2017.
- ↑ Abo R (September 2016). "Optimized photodegradation of Bisphenol A in water using ZnO, TiO2, and SnO2 and photocatalysts under ultraviolet radiation as a decontamination procedure". Drinking Water Engineering and Science. 9 (2): 27–35. doi:10.5194/dwes-9-27-2016.
- ↑ Fox JE, Gulledge J, Engelhaupt E, Burow ME, McLachlan JA (June 2007). "Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants". Proceedings of the National Academy of Sciences of the United States of America. 104 (24): 10282–7. Bibcode:2007PNAS..10410282F. doi:10.1073/pnas.0611710104. PMC 1885820. PMID 17548832.
- ↑ Sun H, Wang L, Zhou Q (January 2013). "Effects of bisphenol A on growth and nitrogen nutrition of roots of soybean seedlings". Environmental Toxicology and Chemistry. 32 (1): 174–80. doi:10.1002/etc.2042. PMID 23109293.
- ↑ Drewes JE, Hemming J, Ladenburger SJ, Schauer J, Sonzogni W (2005). "An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements". Water Environment Research. 77 (1): 12–23. doi:10.2175/106143005x41573. PMID 15765931.
- ↑ Klecka GM, Staples CA, Clark KE, Van der Hoeven N, Thomas DE, Hentges SG (August 2009). "Exposure analysis of bisphenol A in surface water systems in North America and Europe". Environmental Science & Technology. 43 (16): 6145–50. Bibcode:2009EnST...43.6145K. doi:10.1021/es900598e. PMID 19746705.
- ↑ "Bisphenol A Fact Sheet". Government of Canada. Archived from the original on 23 April 2011. Retrieved 1 February 2012.
- ↑ "Bisphenol A Action Plan" (PDF). U.S. Environmental Protection Agency. 29 March 2010. Retrieved 12 April 2010.
- ↑ Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR (2009). "A critical analysis of the biological impacts of plasticizers on wildlife". Philosophical Transactions of the Royal Society B: Biological Sciences. 364 (1526): 2047–62. doi:10.1098/rstb.2008.0242. PMC 2873012. PMID 19528055.
- ↑ Churchwell MI (May 2014). "Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats". Toxicological Sciences. 139 (1): 4–20. doi:10.1093/toxsci/kfu021. PMC 4038784. PMID 24496641.
- ↑ "Bisphenol A (BPA) Information for Parents". Hhs.gov. 15 January 2010. Retrieved 23 October 2011.
- ↑ Biello D (19 February 2008). "Plastic (not) fantastic: Food containers leach a potentially harmful chemical". Scientific American. 2. Retrieved 9 April 2008.
- ↑ "Bisphenol A (BPA): Use in Food Contact Application". FDA.gov. Food and Drug Administration. November 2014. Retrieved 21 June 2018.
- ↑ "COMMISSION DIRECTIVE 2011/8/EU". Official Journal of the European Union.
amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles
- ↑ "NCSL Policy Update: State Restrictions on Bisphenol A (BPA) in Consumer Products". NCSL.org.
- ↑ "NCSL Policy Update: State Restrictions on Bisphenol A (BPA) in Consumer Products". NCSL. Retrieved 20 November 2019.
- ↑ "Senate Bill 70". Delaware General Assembly. Retrieved 19 November 2019.
- ↑ "NCSL Policy Update: State Restrictions on Bisphenol A (BPA) in Consumer Products". ncsl.org. Retrieved 20 November 2019.
- ↑ "NCSL Policy Update: State Restrictions on Bisphenol A (BPA) in Consumer Products". NCSL.org. Retrieved 20 November 2019.
Further reading
- "Products with Bisphenol-A (BPA)". FactsAboutBPA.org.
- Kabiersch G, Rajasärkkä J, Ullrich R, Tuomela M, Hofrichter M, Virta M, Hatakka A, Steffen K (April 2011). "Fate of bisphenol A during treatment with the litter-decomposing fungi Stropharia rugosoannulata and Stropharia coronilla". Chemosphere. 83 (3): 226–32. Bibcode:2011Chmsp..83..226K. doi:10.1016/j.chemosphere.2010.12.094. PMID 21295326.
- Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, Chahoud I, Crain DA, Farabollini F, Guillette LJ, Hassold T, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Laufer H, Marcus M, McLachlan JA, Nadal A, Oehlmann J, Olea N, Palanza P, Parmigiani S, Rubin BS, Schoenfelder G, Sonnenschein C, Soto AM, Talsness CE, Taylor JA, Vandenberg LN, Vandenbergh JG, Vogel S, Watson CS, Welshons WV, Zoeller RT (March 2009). "Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A". Environ. Health Perspect. 117 (3): 309–15. doi:10.1289/ehp.0800173. PMC 2661896. PMID 19337501.