Ansofaxine
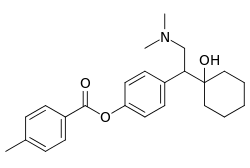
Toludesvenlafaxine (former developmental code names LY03005, LPM570065), also known as ansofaxine or as 4-methylbenzoate desvenlafaxine, is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) which is under development by Luye Pharma Group for the treatment of major depressive disorder (MDD).[1][2][3][4] It is described as an SNDRI and prodrug to desvenlafaxine.[2] However, unlike desvenlafaxine, which has in vitro IC50 values of 53 nM and 538 nM for inhibition of serotonin and norepinephrine reuptake, respectively, toludesvenlafaxine has respective in vitro IC50 values of 723 nM, 763 nM, and 491 nM for serotonin, norepinephrine, and dopamine reuptake inhibition.[2] As of July 2018, the drug is in preregistration for MDD in the United States, the European Union, Japan, and China.[1] In December 2018 the drug is in phase III trial in the United States and China.[5] In March of 2020 the FDA accepted Luye Pharma's new drug application for ansofaxine.[6] In December 2021 Luye Pharma released results from phase II clinical trials on ansofaxine. [7] Luye Pharma Group has issued the brand name Ruoxinlin for the upcoming medication Ansofaxine.[8]
 | |
| Clinical data | |
|---|---|
| Other names | LY03005; LPM570065; 4-Methylbenzoate desvenlafaxine hydrochloride |
| Routes of administration | By mouth |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H31NO3 |
| Molar mass | 381.516 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- "Toludesvenlafaxine extended release - Luye Pharma - AdisInsight".
- Zhang R, Li X, Shi Y, Shao Y, Sun K, Wang A, Sun F, Liu W, Wang D, Jin J, Li Y (2014). "The effects of LPM570065, a novel triple reuptake inhibitor, on extracellular serotonin, dopamine and norepinephrine levels in rats". PLOS ONE. 9 (3): e91775. Bibcode:2014PLoSO...991775Z. doi:10.1371/journal.pone.0091775. PMC 3948889. PMID 24614602.
- Dale, Elena; Bang-Andersen, Benny; Sánchez, Connie (2015). "Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs". Biochemical Pharmacology. 95 (2): 81–97. doi:10.1016/j.bcp.2015.03.011. ISSN 0006-2952. PMID 25813654.
- Completion of Phase 1 Clinical Studies of Ansofaxine Hydrochloride Extended Release Tablets (LY03005) in the U.S.
- "Luye Pharma's Global Strategy For CNS-Focused Products Gathers Pace: Rotigotine Extended Release Microspheres Submitted to Japan's PMDA for Clinical Trials Approval - Press Releases - Luye Pharma Group". www.luye.cn. Retrieved 2022-02-08.
- "NDA Filing for Luye Pharma's Antidepressant Drug LY03005 Accepted by the U.S. FDA - Press Releases - Luye Pharma Group". www.luye.cn. Retrieved 2022-02-08.
- "Luye Pharma Releases Top-Line Results from a Phase II Clinical Trial of Its New Antidepressant Ansofaxine Hydrochloride Extended-Release Tablets". BioSpace. Retrieved 2022-02-08.
- "R&D - Luye Pharma Group". www.luye.cn. Retrieved 2022-10-06.